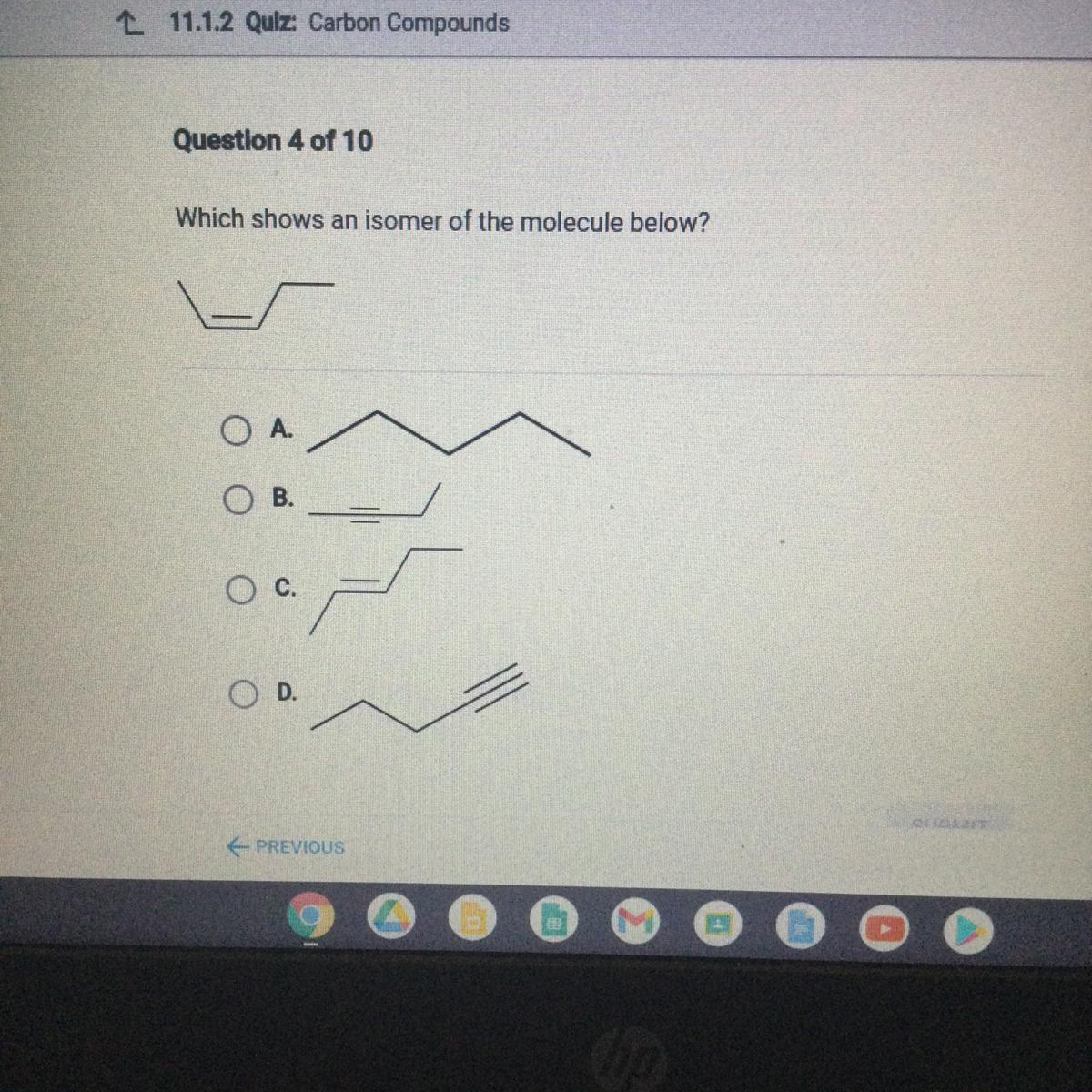
Answers
Answer: C
Explanation:
Don’t trust the other person it’s not A
*view photo for answer*
pay attention! the answer choices aren't always in the same order!
Related Questions
Neptune _____.
is a terrestrial planet
has a nearly horizontal axis
was discovered by mathematical calculations
is the largest planet in the solar system
Answers
Answer:
C. Was Discovered by Mathematical calculations
Explanation:
Terrestrial Planets are Inner Planets (Mercury,Venus,Earth,Mars) and Neptune is an ice giant or gas giant, Neptune is also not the largest planet in the solar system, I believe that's Jupiter. I don't know for sure if Neptune has a horizontal axis but my best guess is C. I hope this helps! :^)
Lead, gold mercury, aluminum which one has the lowest heat capacity?
Answers
Describe and show an example of valence electrons. What is a common trend you can remember to help you understand valence electrons?
Answers
Explanation:
valency from right to left in a Periodic Table in same period first increases then decreases and from top to bottom it is same for a same group
If a book has a weight of 23.2 N on Earth, what is its mass?
Answers
Answer: Mass is 2,37 kg
Explanation: Weight G = mg, and g = 9.81 m/s² on Earth.
m = W/g = 23.2 N / 9.81 m/s²
A man needs to lift a heavy box on the back of a truck or push up a ramp or lift it straight up from the ground to use around he won't have to move the box over a longer distance what did is the benefit of using a ramp to get the box
Answers
Awnser all of them if you can its really EASY im feelin generous today 30 pts
Answers
Answer: 1. Synthetic Substance or Synthetic compound 2. Oil 3. Biofuels 4. Salt and Sugar 5. medicine 6. Synthetic 7. Background Knowledge and Visualizing
Explanation: Hope this helps! Good luck!
Answer:
1= synthetic substance
2= natural gas
3= alcohol
4=artificial musks
5= medicine
6= synthetic
7=Questioning the Text
Explanation: If my answers are correct please mark me as a brainlist.
A 125.0-g sample of a metal heated to 100.0 ∘C and placed in a calorimeter that contains 250.0 g of water. The temperature rises from 24.3 ∘C to 27.2 ∘C. What is the specific heat capacity of the metal? Ignore the calorimeter in your analysis. Group of answer choices
Answers
Answer:
0.333J/g°C is the specific heat of the metal
Explanation:
The heat that the metaal gives is equal to the heat that water is absorbing. The equation is:
S(metal)*ΔT(metal)*Mass(metal) = S(H2O)*ΔT(H2O)*Mass(H2O)
Where S is specific heat, ΔT is change in temperature and mass the mass in grams of the metal and water.
Replacing:
S(metal)*(100.0°C-27.2°C)*125.0g = 4.184J/g°C*(27.2°C-24.3°C)*250.0g
S(metal) = 4.184J/g°C*(27.2°C-24.3°C)*250.0g / (100.0°C-27.2°C)*125.0g
S(metal) = 0.333J/g°C is the specific heat of the metal
The equilibrium of 2H2O(g) 2H2(g) + O2(g) at 2,000 K has a Keq value of 5.31 x 10-10. What is the Keq expression for this system?
A) [H2}^2[O2} / [H2O]^2
B) [H2][O2] / [H2O]
C) [H2O]^2 / [H2]^2[O2]
D) [H2O]^2 / [H2][O2]
Answers
Answer:
B
Explanation:
I don't know how to explain this but you get it
M
Mass can be measured in local units like dharni pau eto. Discuss the advantages of using
standard units of mass over the local units of mass. (Any two points)
N2 Points)
soup answer
Answers
Answer:
1.They are reliable.
2.They dont vary from person to person
calculate the ph of the solution [oh-] = 1x 10-11 m
a. 11.0
b. 12.0
c. 2.7
d. 3.0
Answers
Answer:
d. 3.0
Explanation:
First we calculate the pOH of the solution, using the following formula:
pOH = -log[OH⁻]pOH = -log[1x10⁻¹¹ M]pOH = 11.0Using the pOH we can calculate the pH of the solution, keeping in mind the relationship between pH and pOH:
pH = 14.0 - pOHpH = 14.0 - 11.0pH = 3.0The answer is option d.
A reaction yield 6.26 g of a product what is the percent yield if the theoretical yield is 18.81 g
Answers
Explanation:
remember the equation percentage yield = actual/theoretical yield
so 6.26/18.81 X 100 gives u 33.28017012 so to 3 SF ot would be 33.3%
hope this helps:)
The statement, that describes the percent yield is 33.280 g.
What is percent yield?The percent yield is the ratio of actual yield to theoretical yield. It is computed by dividing the experimental yield by the theoretical yield and multiplying the result by 100 percent. If the actual and theoretical yields are equal, the percent yield is one hundred percent.
To express the efficiency of a reaction, use the following formula: percent yield
= (actual yield/theoretical yield) x 100
= (6.26 g/ 18.81 g) x 100
= 33.280 g
Hence the correct answer is 33.280 g.
Learn more about percent yield here
https://brainly.in/question/5487028
#SPJ2
Hikers have noticed that a sealed bag of potato chips puffs up when taken to the top of a mountain. Suppose that at the valley floor below, the air pressure is 1.0 atm, temperature is 25°C, and the volume of the chip bag is 0.985 L. At the top of the mountain, the temperature is 22°C and the chip bag has puffed up to 1.030 L.
What is the air pressure on top of the mountain?
__ atm
Answers
Answer:
0.95
Explanation:
trust
which substance has a mass is 13.5 grams, and its volume is 5 cm 3.
Answers
Answer:
Substance is Aluminium
Explanation:
We are given;
Mass; m = 13.5 grams
Volume; V = 5 cm³
Formula for density is;
density = m/V
Density = 13.5/5
Density = 2.7 g/cm³
From the table attached, we can see that the element with a corresponding density of 2.7 is Aluminium
Which best describes the purpose of a fever?
Answers
Answer:
when you have a fever your body is trying to fight off a virus or some other kind of bad Bacteria
Explanation:
I think this because everyone has at least had a fever when they were sick and when you are sick you are likely to have a fever because your body is fighting off a bad bacteria
Please help is for my final An example of a chemical change is-
evaporation of seawater
о
sublimation of dry ice (CO2)
melting of aluminum
burning wood reducing it to ashes
Answers
Explanation:
Souring of milk
Baking of cake
How many moles are in 2.8x10^24 atoms of silicon?
Answers
Answer:
4.6 mol Si
General Formulas and Concepts:
Atomic Structure
Reading a Periodic TableMolesAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:
Step 1: Define
[Given] 2.8 × 10²⁴ atoms Si
[Solve] moles Si
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
[DA] Set up: [tex]\displaystyle 2.8 \cdot 10^{24} \ atoms \ Si(\frac{1 \ mol \ Si}{6.022 \cdot 10^{23} \ atoms \ Si})[/tex][DA] Divide [Cancel like units]: [tex]\displaystyle 4.64962 \ mol \ Si[/tex]Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
4.64962 mol Si ≈ 4.6 mol Si
Consider the reaction.
2HF(g) +
H2(g) + F2(g)
At equilibrium at 600 K, the concentrations are as follows.
[HF] = 5.82 x 10-2 M
[H2] = 8.4 x 10-3M
[F2] = 8.4 x 10-3M
What is the value of Kea for the reaction expressed in scientific notation?
O 2.1 x 10-2
102.1 x 102
1.2 x 103
0 1.2 x 10-3
Answers
Answer:
0.021
Explanation:
Step 1: Write the balanced equation
2 HF(g) ⇄ H₂(g) + F₂(g)
Step 2: Calculate the value of the concentration equilibrium constant
The concentration equilibrium constant (K) is equal to the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients.
K for this reaction is:
K = [H₂] × [F₂] / [HF]²
K = (8.4 × 10⁻³) × (8.4 × 10⁻³) / (5.82 × 10⁻²)² = 0.021
Which of these is a polar molecule?
1. SH2
2. CO2
3. F2
Answers
Which of the following is an example of a molecular element?
a.NaCl b. CO2 c. Na d. O2
Answers
Answer:
oxygen (O2) is a molecular element
Explanation:
Molecular compound are chemical compounds that take the form of discrete molecules.
atomic element is an element which occurs independently as atoms and can react with other elements to form a compound
Ionic compounds are the compound which on dissolution form ions in solution
Mixture is the composition of different compounds
PLZ SOMEONE HELP I’LL MARK AS BRAINLIEST!!!
In an experiment 10 grams of a substance has a volume of 24 cm? What is the
substances' density ?
Answers
Answer:
[tex]d=0.417\ g/cm^3[/tex]
Explanation:
Given that,
Mass, m = 10 g
Volume, V = 24 cm³
We need to find the density of the substance. We know that, the density of a substance is given by :
Density = mass/volume
So,
[tex]d=\dfrac{10\ g}{24\ cm^2}\\\\d=0.417\ g/cm^3[/tex]
So, the density of the substance is [tex]0.417\ g/cm^3[/tex].
What is the acceleration of a 7 kg
mass being pulled by a 56 N force?
Answers
Answer:
8 meters per second per second
Explanation:
Newton's second law: [tex]f=ma[/tex], force = mass multiplied by acceleration.
Therefore 56 / 7 = acceleration = 8
What are the three parts of modern cell theory. Choose all that apply.
A.) The cell is the smallest living unit in organisms
B.) All cells come from other pre-existing cells
C.) All living things are made of cells
D.) All organisms are multicellular
Answers
Answer:
a,b,c
Explanation:
Answer:
C
...................
How many moles are in a solution with a concentration of 5 M and a volume of 0.25 L?
Answers
Answer:
160 mL
Explanation:
Mr. Ragusa is approached in his lab by one of his students named Hassan. Hassan had found a bottle of nitric acid that did not have a labeled molarity and wanted Mr. Ragusa to help him find it's molarity. Mr. Ragusa filled a buret to 4.85 mL with 0.30 M NaOH. Mr. Ragusa added 5 mL of the acid to a flask and added some phenolphthalein. Mr. Ragusa hit the titration spot on, and the final volume in the buret was 43.69 mL. What is the concentration of the Nitric Acid?
Round your answer to 2 decimal places. Include a unit in your answer. (ex: 1.2M)
HNO3 + NaOH --> NaNO3 + H2O
Answers
Answer:
2.33M
Explanation:
1. First, make sure the equation is balanced. In this case it would be HNO3+NaOH --> NaNO3 + H2O. (it is already balanced here.)
2. Find the initial volume, final volume, the volume of acid, volume of NaOH.
a. Initial Volume: 4.85 mL
b. Final Volume: 43.69
c. Acid (HNO3) Volume: 5 mL
d Volume NaOH: Initial V - Final V --> 43.69 mL - 4.85 mL = 38.84 mL NaOH
3. Find moles of NaOH reacted using the volume from D. Convert this volume into Liters. Multiply the volume of NaOH by molarity of NaOH given to you before. (.30 M NaOH)
a. x mol NaOH/ .03884 L = .30 mol NaOH/ 1 mol = .011652 mol NaOH
4. Now find the moles of acid. The acid given to you in the equation is HNO3. The .011652 mol NaOH is from the moles reacted that you just found. The moles on the right side would be the co-efficient in front of the elements in the equation. Since this equation does not have any coefficients (it's already balanced), it would just be 1.
a. .011652 mol NaOH/ x mol HNO3 = 1 mol NaOH/ 1 mol HNO3.
x = .011652 mol HNO3
5. Now find the concentration, also known as molarity. Divide the .011652 mol by the volume of acid you have been given (5 mL). Before using 5 mL, divide it by 1000 to get the Liter form.
a. .011652 mol HNO3/ .005 L = 2.3304 M HNO3
I hope you understand now. God bless!
The name for the process that causes fertile land to become dirt-like is??
Deforestation
Urbanization
Construction
Desertification
Answers
Answer:
Desertification?
Explanation:
Desertification is when fertile lands become desert like
HURRY PLEASE
What volume is occupied by 3.12 moles of a gas when the pressure is 88.4 kPa at a temperature of 19℃?
Answers
Answer:
The Combined Gas Law shows that the pressure of a gas is inversely proportional to volume and directly proportional to temperature. Avogadro's Law shows that volume or pressure is directly proportional to the number of moles of gas. Putting these together leaves us with the following equation:
P1×V1T1×n1=P2×V2T2×n2(11.9.1)
As with the other gas laws, we can also say that (P×V)(T×n) is equal to a constant. The constant can be evaluated provided that the gas being described is considered to be ideal.
The Ideal Gas Law is a single equation which relates the pressure, volume, temperature, and number of moles of an ideal gas. If we substitute in the variable R for the constant, the equation becomes:
P×VT×n=R(11.9.2)
The Ideal Gas Law is conveniently rearranged to look this way, with the multiplication signs omitted:
PV=nRT(11.9.3)
The variable R in the equation is called the ideal gas constant.
Explanation:
If the graph of y = a x 2 + b x + c does not intersect the x -axis, then what is true about the roots? Both are real roots. Both are real roots. Both are imaginary roots. Both are imaginary roots. One is a real root, and one is irrational. One is a real root, and one is irrational. One is a real root, and one is rational.
Answers
Answer:
Both roots are imaginary roots.
Explanation:
Consider these things:
If we try to solve x²+1 = 0, notice that we aren't able to solve the equation in Real Number system because there are no negative outputs for quadratic function.
Remember that quadratic function has range greater or equal to the max-min value.
x-axis plane represents the solutions of that equation. If a graph intersects x-axis plane then it has a solution.
While a graph that doesn't have any intersects on x-plane, it means that the equation for that graph doesn't have real solutions but imaginary solutions.
As you may notice some of parabola graph has one intersect, two intersects or none. One intersect is one solution to the equation — Two intersects are two solutions of the equation and lastly, no intersects mean that no real solutions and remain only imaginary solution.
What are some of the issues facing the ocean ( give at least 3 )
Answers
Answer:
Pollution
Overpopulation
Destruction of habitats
Explanation:
When two atoms form a molecule, the molecule has lower energy than the two separate atoms. However, when only two atoms are present, they bounce off each other and do not form a molecule. Explain what happens to the energy of the atoms when there are only two atoms and they collide.
DONT GIVE ME A LINK!!
Answers
Answer:
they end up bouncing against each other instead of merging together
Explanation:
what is zinc chemical formula?
Answers
Answer:
Zn+2
Explanation:
I'm sorry if its wrong but have a good one!