Answers
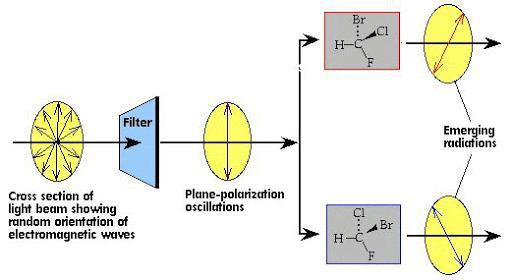
Geometric isomers have the same structural formulas but differ in the arrangement of groups at a single atom, at double bonds, or in rings. ... One of the optical isomers rotates the light in one direction, the other rotates the light in the opposite direction but by the same amount.
Related Questions
A gas has a solubility of 2.45 g/L at a pressure of 0.750 atm. What pressure wold be required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature
Answers
Answer:
1.91 atm
Explanation:
Step 1: Calculate Henry's constant (k)
A gas has a solubility (C) of 2.45 g/L at a pressure (P) of 0.750 atm. These two variables are related to each other through Henry's law.
C = k × P
K = C/P
K = (2.45 g/L)/0.750 atm = 3.27 g/L.atm
Step 2: Calculate the pressure required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature.
We have C = 6.25 g/L and k = 3.27 g/L.atm. The required pressure is:
C = k × P
P = C/k
P = (6.25 g/L)/(3.27 g/L.atm) = 1.91 atm
The blank
and
blank
of atoms are the same on both sides of
a chemical equation.
Please just answer the question directly instead of giving me some weird cryptic answer that I can’t use. I’ve seen this exact question answered before and nobody would give a straight answer.
Answers
Which statement describes the atoms in an element
Answers
Answer:
Atoms are kind of like building blocks.
Explanation:
Think of atoms as kind of like a mix between paint pigments and legos. The more legos that you have, the bigger the molecule but keep in mind that there are many different kinds of legos that all can come together to build numerous things. I hope that I answered your question and i apoligize if I didn't.
How to solve for K when given your anode and cathode equations and voltage
Answers
Answer:
See Explanation
Explanation:
In thermodynamics theory the Free Energy (ΔG) of a chemical system is described by the expression ΔG = ΔG° + RTlnQ. When chemical system is at equilibrium ΔG = 0. Substituting into the system expression gives ...
0 = ΔG° + RTlnKc, which rearranges to ΔG° = - RTlnKc. ΔG° in electrochemical terms gives ΔG° = - nFE°, where n = charge transfer, F = Faraday Constant = 96,500 amp·sec and E° = Standard Reduction Potential of the electrochemical system of interest.
Substituting into the ΔG° expression above gives
-nFE°(cell) = -RTlnKc => E°(cell) = (-RT/-nF)lnKc = (2.303·R·T/n·F)logKc
=> E°(cell) = (0.0592/n)logKc = E°(Reduction) - E°(Oxidation)
Application example:
Calculate the Kc value for a Zinc/Copper electrochemical cell.
Zn° => Zn⁺² + 2e⁻ ; E°(Zn) = -0.76 volt
Cu° => Cu⁺² + 2e⁻ ; E°(Cu) = 0.34 volt
By natural process, charge transfer occurs from the more negative reduction potential to the more positive reduction potential.
That is,
Zn° => Zn⁺² + 2e⁻ (Oxidation Rxn)
Cu⁺² + 2e⁻ => Cu° (Reduction Rxn)
E°(Zn/Cu) = (0.0592/n)logKc
= (0.0592/2)logKc = E°(Cu) - E°(Zn) = 0.34v - (-0.76v) = 1.10v
=> logKc = 2(1.10)/0.0592 = 37.2
=> Kc = 10³⁷°² = 1.45 x 10³⁷
which molecule is butene
Answers
Answer:
Option C is the answer
Butene, also called Butylene, 4 isomeric compound belonging to the series of olefinic hydrocarbons. The chemical formula is C4H8, option c is correct.
What are the 4 isomers of butene?Butene, also called Butylene, four isomeric Combinations belong to the series of olefinic hydrocarbons. The chemical formula is C4H8.
The isomeric forms are 1-butene, cis-2-butene, trans-2-butene, and isobutylene.
Thus, option "C" is correct. the chemical formula is C4H8.
To learn more about Butene click here:
https://brainly.com/question/13186233
the correct order of increasing ionisation energy of Na,Al,Si and Mg elements is?
Answers
Removal of an electron from stable, fully filled orbital requires more energy than removal of electron from partially filled orbital. Thus, ionisation enthalpy for Mg is greater than ionisation enthalpy for Al. So, the correct order of first ionization enthalpies is: Na<Mg>Al<Si.
How many moles of nitrogen are required to produce 13.5 g of NH 3?
Answers
Answer:
number of moles of (N) = 0.794 moles
Explanation:
From the given information:
no of moles of nitrogen (N) = (unknown)???
mass of nitrogen = 13.5 g
molar mass of NH3 = 14 +( 1 × 3) = 17 g/mol
To calculate the no of moles of N, we have:
number of moles of (N) = mass of N/molar mass
number of moles of (N) = 13.5 g/17 g/mol
number of moles of (N) = 0.794 moles
Which
gas is exchanged in the air sac of
longs? -
oxygen and nitrogen
oxygen and carbondioxide
O nitrogen and coarbondioxide
oxygen and hydrogen
O
Other:
Answers
Answer:
oxygen and carbon dioxide.
hope this helps you
A solution with a pH of 5.30 has a H+ concentration of
Answers
Answer:
5.01 x 10^-6 M
Explanation:
PH= -log [H+]
[H+] = 10^-PH
Question 2 (1 point)
An object's gravitational force depends primarily on the object's
a
density
mass
b
oc
momentum
Answers
Answer: An object's gravitational force depends primarily on the object's mass.
Explanation:
According to the Universal law of gravitation, every object whether is it is having large r small mass tends to exert a force on every other object, therefore this force is known as gravitational force.
Formula to calculate gravitational force is as follows.
[tex]F = \frac{G \times m_{1} \times m_{2}}{r^{2}}[/tex]
where,
F = Gravitational force
G = Gravitational constant = [tex]6.674 \times 10^{-11} m^{3}/kg s^{2}[/tex]
[tex]m_{1}[/tex] = mass of object 1
[tex]m_{2}[/tex] = mass of object 2
r = distance between the centers of the objects
Therefore, it means that gravitational force of an object primarily depends on an object's mass.
Thus, we can conclude that an object's gravitational force depends primarily on the object's mass.
When water is boiling in a pot, heat energy is being transferred throughout the water by which type of heat transfer?
O Convection
O Conduction
O Radiation
Answers
Answer:
it would be Convection.
Which of the following are reasons the ocean marine biomes are a challenging place for organisms to live? Choose all that apply.
Question 13 options:
some areas are extremely dark and deep
high waves can be rough and powerful
all of the organisms have to live without sunlight
organisms have to be able to live in extremely salty conditions
Answers
In an ecosystem, the reason that ocean marine biomes are a challenging place for organisms to live because they have to be able to live in extremely salty conditions.
What is an ecosystem?Ecosystem is defined as a system which consists of all living organisms and the physical components with which the living beings interact. The abiotic and biotic components are linked to each other through nutrient cycles and flow of energy.
Energy enters the system through the process of photosynthesis .Animals play an important role in transfer of energy as they feed on each other.As a result of this transfer of matter and energy takes place through the system .Living organisms also influence the quantity of biomass present.By decomposition of dead plants and animals by microbes nutrients are released back in to the soil.
Learn more about ecosystem,here:
https://brainly.com/question/13979184
#SPJ3
Calculate the volume of 0.250 M H2SO4 that contains 0.00255 mol H2SO4.
Answers
Answer:
0.0102 L or 10.2 mL
Explanation:
From the question given above, the following data were obtained:
Molarity = 0.250 M
Mole = 0.00255 mole
Volume =?
Molarity is simply defined as the mole of solute per unit litre of the solution. Mathematically, it is expressed as:
Molarity = mole / Volume
With the above formula, we can obtain the volume as follow:
Molarity = 0.250 M
Mole = 0.00255 mole
Volume =?
Molarity = mole / Volume
0.250 = 0.00255 / volume
Cross multiply
0.250 × volume = 0.00255
Divide both side by 0.250
Volume = 0.00255 / 0.250
Volume = 0.0102 L
Covert 0.0102 L to mL.
1 L = 1000 mL
Therefore,
0.0102 L = 0.0102 L × 1000 / 1 L
0.0102 L = 10.2 mL
Thus, the volume is 0.0102 L or 10.2 mL
1. How many grams of sodium carbonate must be weighed out in order to make 2.5 kg of a 35.0%
(w/w) solution?
Answers
Answer:
... x M x V. • Example: Prepare 800 mL of 2 M sodium chloride. ... solutions are indicated by w/v% and are defined as the grams of solute per ...
15 pages·906 KB
what cause a mass defect?
○A. The mass of a nucleus is larger than it should be.
○B. The mass of a nucleus cannot be accurately measured.
○C. Mass is converted to the energy binding a nucleus together.
○D. Mass is lost when a particle is released in a reaction.
Answers
Answer: Mass is converted to the energy binding a nucleus together.
Explanation: a p e x
Mass is converted to the energy binding a nucleus together cause a mass defect.Hence , Option (C) is correct.
What is Mass defect ?
The actual atomic mass is less than the predicted mass calculated by adding the masses of nucleons.
This additional mass is accounted for by binding energy that is released when a nucleus is formed.
When a nucleus is formed, some of the mass is converted to energy and this results in the mass defect.
Therefore, Mass is converted to the energy binding a nucleus together cause a mass defect. Hence , Option (C) is correct.
Learn more about mass defect here ;
https://brainly.com/question/11624098
#SPJ2
Uranium is an element with three naturally occurring isotopes: 238U, 235U, and 234U. This means that 238U, which has a mass number of 238 has _______more than 235 u which has a mass number of 235.
Answers
Answer:
The correct answer is - neutrons.
Explanation:
Uranium has various isotopes found naturally that are three 238U, 235U, and 234U. Uranium has an atomic number of 92 which means there are 92 protons and 92 electrons in the atomic structure.
Isotopes have the same number of protons but a different number of neutrons that can vary from 141 to 146. U-238 has 146 neutrons in the nucleus, whereas 235 U has 143 neutrons.
The U- 238 has more neutrons than U- 235. Atomic mass is the sum total of the nucleons or protons and neutrons.
What are Isotopes?
They are the different variants of the same molecules which have the same number of protons but the different number of neutrons.
Atomic mass is the sum total of the nucleons or protons and neutrons. The atomic number of Uranium is 92, the rest of the mass comes from neutrons.
Therefore, the U- 238 has more neutrons than U- 235.
Learn more about Isotopes:
https://brainly.com/question/9099776
PLZ ANSWER QUICK I AM IN TIMER HELP WILL GIVE BRAINLIEST
Which is greater, the moon's period of rotation or its period of revolution?
They are equal.
the moon's revolution period around Earth
Neither are known.
the moon's rotational period
Answers
Answer:
B
Explanation:
Why would you want to slow down a chemical reaction?
Answers
Answer:
We often want to decrease the rates of certain reactions rather than speeding them up. For example, to prolong the shelf lives of certain foods, the chemical reactions by which they spoil must be slowed down.
When looking across the periodic table from left to right, which of these groups are the first to contain nonmetals?
group 2
group 7
group 14
group 18
Answers
Answer:
The first group to contain a non-metal is Group 18.
mplete the following Charles' Law calculations. If the current temperature is 25 degrees C and you have a 2L balloon, identify the new volume of the balloon if you increase the temperature to 30 degrees C. Remember to change Celsius to Kelvin: K= C + 273.
Answers
Answer:
New volume = 2.03 L
Explanation:
Given that
Initial temperature, T₁ = 25°C = 298 K
Initial volume, V₁ = 2L
Final temperature, T₂ = 30°C = 303 K
We need to find the final volume. The mathematical form of Charle's law is given by :
[tex]\dfrac{V_1}{V_2}=\dfrac{T_1}{T_2}\\\\V_2=\dfrac{V_1T_2}{T_1}[/tex]
Put all the values,
[tex]V_2=\dfrac{2\times 303}{298}\\\\V_2=2.03\ L[/tex]
so, the new volume is equal to 2.03 L.
what is chemical name of ghee
Answers
Answer. Chemical formula of ghee: (C17H33 Coo)3 C3H5 • Chemical Reaction: (C17H31Coo)3 C3H5 + 3H2 (C17H33 Coo)3 C3H5
Suppose you have a solution that might contain any or all of the following cations: Cu 2, Ag , Ba 2, and Mn2 . The addition of HBr causes a precipitate to form. After the precipitate is filtered off, H2SO4 is added to the supernate, and another precipitate forms. This precipitate is filtered off, and a solution of NaOH is added to the supernatant liquid until it is strongly alkaline. No precipitate is formed. Which ions are present in each of the precipitates
Answers
Answer:
Ag⁺, Ba²⁺,
Explanation:
We can solve this question using the solubility rules:
When an Halide as Br- is added to a solution, the ions that can be precipitate are Ag⁺, Hg₂²⁺ and Pb²⁺.
That means the first ion present is Ag⁺
When sulfates, SO₄²⁻ are added, the ions that precipitates are: Ag⁺, Ca²⁺, Sr²⁺, Ba²⁺, Hg₂²⁺ and Pb²⁺
The second ion present is Ba²⁺
Hydroxides of Cu²⁺ and Mn²⁺ are insolubles but as no precipitate are formed when the solution is strongly alkaline those ions are not present.
7. What is the mass of a piece of copper (Cu) that undergoes a 25.0 °C
temperature change with the loss of 428 J of energy?
Answers
Answer:
0.0428 kg or 42.8 g
Explanation:
Applying,
Q = cmΔt.............. Equation 1
Where Q = heat lost of heat gained, c = specific heat capacity of copper, m = mass of copper, Δt = temperature change.
make m the subject of the equation above
m = Q/cΔt.................. Equation 2
From the question,
Given: Q = 428 J, Δt = 25 °C
Constant: c = 400 J/kg.°C
Substitute these values into equation 2
m = 428/(25×400)
m = 0.0428 kg
m = 42.8 g
How many grams of Ni are formed from 55.3 g of Ni2O3?
2Ni2O3(s)⟶4Ni(s)+3O2(g)
Answers
Answer:
39.2 g
Explanation:
2Ni₂O₃(s) ⟶ 4Ni(s) + 3O₂(g)First we convert 55.3 grams of Ni₂O₃ into moles of Ni₂O₃, using its molar mass:
55.3 g ÷ 165.39 g/mol = 0.334 mol Ni₂O₃Then we convert 0.334 moles of Ni₂O₃ into moles of Ni, using the stoichiometric coefficients of the balanced reaction:
0.334 mol Ni₂O₃ * [tex]\frac{4molNi}{2molNi_2O_3}[/tex] = 0.668 mol NiFinally we calculate how much do 0.668 Ni moles weigh, using the molar mass of Ni :
0.668 mol Ni * 58.69 g/mol = 39.2 gWhich example is the site of oxidation when the dry cell is operating?
solid zinc layer
cardboard separator
graphite rod
paste
Answers
A dry cell is one type of electric battery which is generally used for the home and portable electronic devices. A battery is a device which consists of one or more electrochemical cells. The site of oxidation occurs in solid zinc layer. The correct option is A.
What is a dry cell?A dry cell is defined as an electrochemical cell which consists of low moisture immobilized electrolytes in the form of a paste which restricts it from flowing. It is developed by the German scientist Carl Gassner which converts chemical energy into electrical energy.
A dry cell consists of a metal container in which a low moisture electrolyte paste covers the graphite rod or a metal electrode. Generally, the metal container will be zinc whose base perform as the negative electrode (anode).
For a dry cell battery to operate, oxidation will occur from the zinc anode and reduction will take place in the cathode.
Thus the correct option is A.
To know more about dry cell, visit;
https://brainly.com/question/13780178
#SPJ2
Ito ang web browser na kapalit ng Internet Explorer! plsssss help naman ako epp po ito hindi ko po alam
Answers
Answer:
p o e t i c o
Explanation:
no lo entenderias
The sea, on average, has a molarity of 0.599 M NaCl. How many grams of NaCl is this for every 1 liter?
Pls answer
Answers
Answer:
it contains 0.599 g i hope it helps
4. Which of the following process is NOT part of wool extraction?
(a) Shearing (b) Scouring (c) Sorting
(d) Reeling
Answers
Answer:
Reeling is the only process not listed.
The correct answer is option D: Reeling.
Wool is obtained from sheep. The wool so obtained is processed according to the following flow chart;
Shearing → Scouring → Sorting → Dyeing → Straightening, Rolling and Combing
Shearing is the removal of the fleece and thin skin of the sheep. Scouring is the process of washing the hair to remove grease, dust, and dirt. Sorting is the process of differentiating the fibers.
Hence, reeling is not a process in wool extraction.
https://brainly.com/question/9968125
Which of the following is NOT an indication that a chemical reaction has taken place?
Answers
Answer:
WHERE ARE THE CHOICES?
PLEASE MAKE SURE THAT YOUR QUESTION IS RIGHT BEFORE POSTING IT シ︎
Change of state of matter is not an indication that a chemical change has taken place.
What is matter?Matter is a substance which is made up of various types of particles which occupy space and have inertia . All living things and objects are made up of various types of particles that occupy space and have inertia .
Depending on temperature and other factors matter is able to exist in different phases. Most common of which are solid, liquid and gas. Matter can exist in more than one state depending on the temperature and pressure .
State of matter can be changed by heating or cooling and even by changing the applied pressure.When a state changes matter does not break rather its state changes though its chemical composition remains same.
Physical characteristics of matter are shape, color, size and temperature. Every matter is made up of elements which cannot be broken down further by ordinary chemical reactions.
Learn more about matter,here:
https://brainly.com/question/12972782
#SPJ6
to the nearest gram, what is the mass of of one spoonfull of sugar? g
Answers
Answer:
the mass of one spoonful of sugar to nearest gram is 10g