Answers
The correct answer is
C. -1108.7 kJ/mol rxn
From the table values, the standard Gibbs free energy change for the formation of POCl₃ is -1108.7 kJ/mol
What is Gibbs free energy ?We can calculate ΔG° for the reaction using the following formula:
ΔG° = ΣnΔG°f(products) - ΣnΔG°f(reactants)
where ΔG°f is the standard Gibbs free energy of formation for each compound and n is the stoichiometric coefficient of each compound in the balanced chemical equation.
We can look up the values of ΔG°f for each compound in tables of thermodynamic data. At standard conditions (25°C and 1 atm pressure), the values are:
ΔG°f (POCl₃) = -558.9 kJ/mol
ΔG°f (P₂) = 0 kJ/mol
ΔG°f (O₂) = 0 kJ/mol
ΔG°f (Cl₂) = 0 kJ/mol
Plugging these values into the formula, we get:
ΔG° = (2 mol)(-558.9 kJ/mol) - (1 mol)(0 kJ/mol) - (1 mol)(0 kJ/mol) - (3 mol)(0 kJ/mol)
ΔG° = -1117.8 kJ/mol
Therefore, the correct option is C. -1108.7 kJ/molrxn. Note that this answer differs slightly from the calculated value because the given choices are rounded to one decimal place.
Find more on Gibbs energy:
https://brainly.com/question/29753417
#SPJ2
Related Questions
If you have a solution that is 15 percent by mass of KCl in benzene, what is the new boiling point?
Answers
Answer:
https://www.chegg.com/homework-help/questions-and-answers/1-10-points-solution-15-percent-mass-kcl-benzene-new-boiling-point--901-c-b-921-c-c-821-c--q63751186
Explanation: Thats your answer
5
Which of the following is a factual statement about
hormones?
A They are the sole cause of mood swings
B
They cause boys' and girls' bodies to develop
C
They are a type of blood cell
D They are extremely difficult to deal with
Answers
Answer:
They cause boys' and girls' bodies to develop
SOMEONE HELP ME
1. How many grams of C are present in 4.86 grams of carbon dioxide
?
grams C.
2. How many grams of carbon dioxide contain 1.73 grams of O ?
grams carbon dioxide.
Answers
Answer:
1. 1.33 gram of carbon
2. 2.38g of carbon dioxide
Explanation:
From the given information:
Total amount of CO₂ = 4.86 grams
Atomic mass of C = 12 g/mole
molar mass of CO₂ = 44 g/mole
∴
The mass of the Carbon (C) in grams is:
[tex]= Total\ amount \ of \ CO_2 \times \dfrac{12 \ g/mol}{44 \ g/mol}[/tex]
[tex]= 4.86 \ g \times \dfrac{12 \ g/mol}{44 \ g/mol}[/tex]
= 1.33 gram of carbon
2.
Here, the total amount of CO₂ = unknown
Atomic mass of O₂ = 32 g/mole
molar mass of CO₂ = 44 g/mole
amount of oxygen = 1.73 g
∴
The mass of CO₂ = [tex]Total \ amount \ of \ O_2 \times \dfrac{44 \ g/mol}{32\ g/mol}[/tex]
[tex]=1.73 \times \dfrac{44 \ g/mol}{32\ g/mol}[/tex]
= 2.38 g of carbon dioxide
Can someone plss help me answer those 4 questions by tonight.Thank you !
Answers
Answer:
no problem hey I will help you
240 g of water (specific heat = 4.186 J/g°C, initial temperature = 20°C) is mixed with an
unknown mass of iron (specific heat = 0.444 J/gºC, initial temperature 500°C). When
equilibrium is reached, the system has a temperature of 42°С. Find the mass of iron.
Answers
240 g of water (specific heat = 4.186 J/g°C, initial temperature = 20°C) is mixed with an unknown mass of iron (specific heat = 0.444 J/gºC, initial temperature 500°C). When equilibrium is reached,The answer for this would be 69.6
How do you find final temperature with specific heat?
You use q = mcΔT, but you assume aluminum = water and crack for This the final temperature.
We need to look up heat values (c) for aluminum and h20. This will uses 0.901 for aluminum and 4.18 use water
: (10)(130 - T)(0.901) = (200.0)(T - 25 (6)
Hence, The answer for this would be 69.6.
To learn more about specific heat click here:
https://brainly.com/question/1747943
#SPJ2
How/what do I answer this?--> "heat of the chemical reactions"
like this is the question :/
Answers
Answer:
The heat of reaction is the energy that is released or absorbed when chemicals are transformed in a chemical reaction. It describes the change of the energy content when reactants are converted into products.
Explanation:
Yeah, that would confuse me a bit but then you read it and then you will get that answer above! Have a great rest of your day!
Control rods in nuclear reactors are made of materials that absorb free neutrons in order to
slow down the chain reaction.
True
False
Answers
HELP PLEASE Excluding the noble gas group, how does the number of valence electrons in an element influence it chemical reactivity?
Answers
Answer: A. Elements with intermediate numbers of valence electrons are the least reactive.
Explanation: I Quizzed
Colligative properties of solutions include all of the following except: a. an increase in the osmotic pressure of a solution upon the addition of more solute b. elevation of the boiling point of a solution upon addition of a solute to a solvent c. an increase of reaction rate with increase in temperature d. depression of the freezing pont of a solution upon addition of a solute to a solvent e. depression of vapor pressure upon addition of a solute to a solvent
Answers
Answer:
Option C, an increase of reaction rate with increase in temperature
Explanation:
Colligative properties are as follows
a) Decrease of vapor pressure
b) Increase of boiling point
c) Reduction of freezing point
d) Increase of osmotic pressure
There is no impact on reaction rate and hence it is not a colligative property.
Thus, option c is the right choice
An increase of reaction rate with increase in temperature isn't an example
of colligative properties of solutions
Colligative properties of solutions depend on the ratio of the number of
solutes to that of the solvent(concentration) and not on the nature of the
substances involved.
Examples of colligative properties include vapor pressure lowering, boiling
point elevation, freezing point depression, and osmotic pressure. Increase of
reaction rate with increase in temperature is therefore not an example of
colligative properties of solutions.
Read more on https://brainly.com/question/24260365
is this correct im just asking because my little brother not sure for his answer
Answers
Commercial soaps are mixtures of ionic compounds typically made up of monatomic cations, such as Na and K , and organic polyatomic anions derived from fatty acids. These negatively charged molecular ions are characterized by the presence of hydrocarbon chains which are 12 to 18 carbon atoms long. How hard (solid, insoluble) or soft (liquid, soluble) a soap is depends on the nature of the anions and cations present in the system. Analyze how each of the following factors may affect the hardness or softness of soaps:
1. The nature of the cations. For example, Na* vs Li* vs K.
2. The length of the hydrocarbon chain. For example, 12 carbons (laureate lon), 14 carbons (myristate lon), or 18 carbons (stearate lon).
Answers
Answer:
Following are the solution to the given question:
Explanation:
For question 1:
The sodium soap containing Na+ is strong whereas the softer or liquids were potassium soap.It's hard to use lithium soap.These Na+, K+, and Li+ ions act as the hydrophilic center.Calcium and Magnesium ions could be substituted by hard water with increasing hydrophilicity.For question 2:
The hydrophobicity of its carbon chain increases but one appears weaker with only an increased length.Therefore, the laureate is hard, while the stearate is soft.Which of these equations represent reactions that could be used in constructing an electrochemical c
Check all that apply
CH. +202 C02 +2H,0
Cr + Cu? → Cr?++ Cu
2 Ag*+Fe + 2Ag +Fe2
CI+Ag → AgCI
NH, *H* NH,
RETRY
Answers
Answer:
Cr+Cu2 -> Cr2+Cu
2Ag+Fe->2Ag+Fe2
Explanation: I did the assessment
other two substances present in breathed out air
Answers
Answer:
Gases we Breathe Out
It is the same air that we inhale. ... The amount of inhaled air contains 21% of oxygen and 0.04% of carbon dioxide, while the air we breathe out contains 16.4% of oxygen and 4.4% of carbon dioxide.
Answer:
Inhaled air is by volume 78% nitrogen, 20.95% oxygen and small amounts of other gases including argon, carbon dioxide, neon, helium, and hydrogen. The gas exhaled is 4% to 5% by volume of carbon dioxide, about a 100 fold increase over the inhaled amount.
Please help will give brainliest
Perform the following
mathematical operation, and
report the answer to the correct
number of significant figures.
328 x 0.125 = [?]
Answers
Answer: 41.0
Explanation: When you multiply the two numbers you get 41 but you need to have the same amount of significant numbers as the number in the problem with the least significant numbers. I hope this helps
1st law of motion law of inertia in toy story 2
Answers
pls help i need asap will mark brainlest
Answers
Answer:
B. 50%
Explanation:
2H₂ + CO → CH₃OHFirst we convert the given masses of the reactants into moles, using their respective molar masses:
4 g H₂ ÷ 2 g/mol = 2 mol H₂25 g CO ÷ 28 g/mol = 0.893 mol CO0.893 moles of CO would react completely with (0.893 * 2) 1.786 moles of H₂. As there are more H₂ moles than that, H₂ is the reactant in excess and CO is the limiting reactant.
Now we calculate how many CH₃OH moles would have been formed if all CO would have been consumed:
0.893 mol CO * [tex]\frac{1molCH_3OH}{1molCO}[/tex] = 0.893 mol CH₃OHThen we convert 0.893 moles of CH₃OH into grams, using its molar mass:
0.893 mol CH₃OH * 32 g/mol = 28.57 gFinally we calculate the percent yield:
14 g / 28.57 g * 100% = 49%What is The metric unit for volume ?
Answers
Answer:
milliliters
Explanation:
Is going to be milliliters because in the metric system of measurement,the most common unit of volume are milliliters and liters
The oceanic crust is destroyed at convergent boundaries because it_______________________ *
a) diverges to form a rift valley
b) goes into a subduction zone where it is melted by the hot magma
c) transforms
d) the rock crumbles at an ocean ridges
Answers
Answer: I believe the answer is d) the rock crumbles at an ocean ridges
Explanation:
Hydrogen iodide can decompose into hydrogen and iodine gases. 2HI(g) H2(g) I2(g) K for the reaction is 0.016. Of 0.148 atm of HI(g) is sealed in a flask, what is the pressure of each gas when equilibrium is established
Answers
Solution :
Given :
Hydrogen iodide decomposes to hydrogen and iodine gas
[tex]$2 HI \ \ \ \Leftrightarrow \ \ \ \ H_2 \ \ \\ + \ \ I_2 $[/tex]
I 0.148 0 0
C -2a +a +a
E 0.148-2a a a
We know
[tex]$k_p=\frac{P(H_2)P(I_2)}{P(HI)^2}$[/tex]
[tex]$0.016=\frac{a^2}{(0.148-2a)^2}$[/tex]
[tex]$0.016^{1/2}=\frac{a}{0.148-2a}$[/tex]
[tex]$0.12649=\frac{a}{0.148-2a}$[/tex]
0.0187 = 1.2529 a
a = 0.0149
Therefore
P(HI) = 0.148 - 2a
= 0.148 - 2(0.0149)
= 0.1182 atm
P([tex]$H_2$[/tex]) = a
= 0.0149 atm
P([tex]$I_2$[/tex]) = a
= 0.0149 atm
PLEASE NO BOTS lol
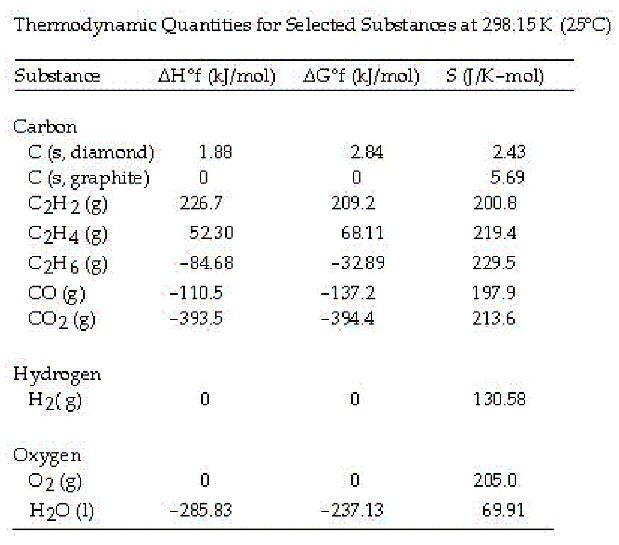
Ethyne or acetylene is also used in cutting torches. The acetylene is combined with pure oxygen producing a flame with a temperature of 6332 °F or 3500 °C. The combustion of acetylene in the presence of excess oxygen yields carbon dioxide and water:
2C2H2 (g) + 5O2 (g) --> 4CO2 (g) + 2H2O (l)
Calculate the value of ΔS° for this reaction.
A. +689.3 J/mol K
B. +432.4 J/mol K
C. -432.4 J/mol K
D. -122.3 J/mol K
Answers
The correct answer is
C. -432.4 J/mol K
Determine the number of moles in 1.5 x 10^25
atoms of iron.
Answers
A scientist wants to investigate several problems. In which of the following situations is using a simulation LEAST suitable for solving a problem?
a. When a scientific study requires performing a large number of trials that need to be conducted very quickly
b. When the solution to the problem requires real-world data inputs that are continually measured at regular intervals.
c. When performing an experiment that would be too costly or dangerous to conduct in the real world
d. When it is considered acceptable to make simplifying assumptions when modeling a real-world object or phenomenon
Answers
Answer:
A
Explanation:
got from another website
The simulation which is least suitable to scientists for solving a problem is when performing an experiment that would be too costly or dangerous to conduct in the real world.
What is scientific approach?Scientific approach is an empirical method of acquiring knowledge and result. This method involved the careful observation of the problem, applying skepticism about the problem and giving cognitive assumptions also.
If a scientist wants to investigate several problems, then they will perform a large no. of trials at regular intervals to get the required result. But if performing of any experiment is dangerous to conduct in the real world then scientists will ignore to solve that problem.
Hence, option (C) is correct.
To know more about scientific approach, visit the below ink:
https://brainly.com/question/497944
A 0.227 mol chunk of dry ice (solid CO2) changes to gas. What is the volume of that gas measured at 27 °C and 740 mmHg?
Answers
Answer:
Explanation:3.2 ft 2 fti2 ft 4 ft ft2
Can someone please help me
Answers
Plants!
No doubt the answer is plants they are one maintaining the temperature, responsible for rains too...
4.
How many parents take part in binary fission?
Answers
Answer:
one parent
Explanation:
As one parent cell divides it into two daughter cells and so on.
Which hand is negatively changed?
A.
B.
C.
D.
Answers
Answer:
I think B
Explanation:
There are more negative ions than positive ions
What is the percent by mass of magnesium in a 1000 g sample of ocean water (solution) that has 1.36 g of magnesium ions?
Answers
Answer:
So the percentage mass of Magnesium in ocean water is 0.13%.
Explanation:
brainliest pls
PLZ HELP ON TIMER WILL GIVE BRAINLIEST
How does technology limit the future of space exploration?
There are too many devices in space interfering with taking correct measurements.
Scientists cannot make contact with older satellites in outer space.
Scientists are able to work both with current and future technology.
Scientists must first develop certain technologies before missions can be completed.
Answers
Answer:
I would put the final answer choice: "Scientists must first develop certain technologies before missions can be completed"
Explanation:
The first option is partially true, but we have ways around it.
The second option is straight-up false.
The third option doesn't make much sense, how can one work with technology that will be developed in the future and doesn't yet exist?
Therefore, the fourth option is the best.
Hope this helps
-cyber
______ is the process of change from a liquid to a gas at temperatures below the boiling
point.
Answers
Answer:
evaporation is the process
Answer:
EvaporationExplanation:
Evaporation is the process of becoming a vapor. The process of extracting moisture as by heat.
Evaporation is the process of change from a liquid to a gas at temperatures below the boiling point.
Therefore, the final answer is evaporation.
If you have 600g of nitroglycerin, how many moles do you have?
help please
Answers
Answer:
600
Explanation:
there's 1 mole in every nitroglycerin
I think