Answers
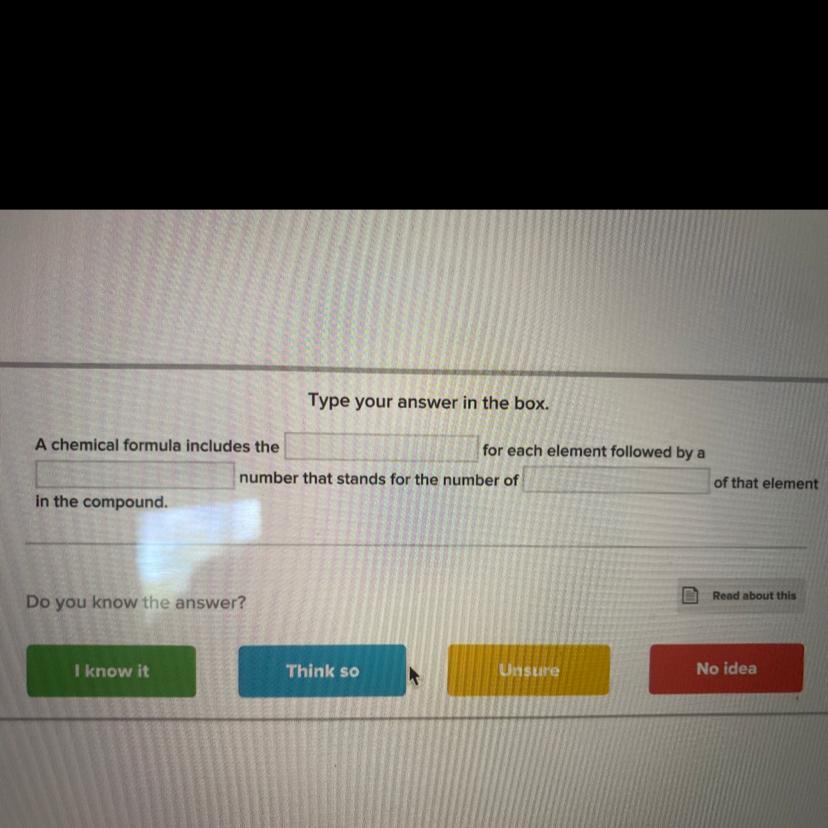
maybe umm think so pick think so
Related Questions
does the moon really change shape? why
Answers
is this correct im just asking because my little brother not sure for his answer
Answers
How does carbonic acid work to maintain blood pH? (Select all that apply.) Check All That Apply When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid can raise or lower the pH of blood. Carbonic acid can raise or lower the pH of blood.
Answers
Answer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Carbonic acid can raise or lower the pH of blood.
Explanation:
A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. The human blood serves as a buffer as it contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3-) which serves to maintain blood pH between 7.35 and 7.45. Other buffering systems in blood exist such as the Hydrogen ion and oxygen gas which affects oxygen binding to haemoglobin, however the carbonic-acid-bicarbonate buffer is the most important buffer for maintaining acid-base balance in the blood.
A buffer solution is made up of an acid and its conjugate base or a base and its conjugate acid. For carbonic acid-bicarbonate buffer, carbonic acid serves as the acid while bicarbonate serves as the base. When a little quantity of a base as hydroxide ions is added to a buffer, the acid reacts with it and remove it from the solution. On the other hand, when a little quantity of an acid as hydrogen ions are added to a buffer, the conjugate base reacts with it and remove it from the solution, thus keeping the pH of the solution fairly constant.
In the carbonic acid-bicarbonate buffer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Thus, carbonic acid can raise or lower the pH of blood.
Carbonic acid work to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.WHAT IS BUFFER SOLUTION:A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. A buffer is made up of an acid and its conjugate base or a base and its conjugate acid. Carbonic acid is an example of buffer that contains an acid with it's conjugate base.This means that, carbonic acid works to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.Learn more about buffers at: https://brainly.com/question/24188850
For the following reaction conditions select the correct statement regarding the reaction below.
OH Cl OTs OTs N TsCl W X Y Z N
a. W is the correct product.
b. X is the correct product.
c. Y is the correct product.
d. Z is the correct product since pyridine acts as a nucleophile.
e. Both X and Y are both formed in this reaction as a racemic mixture
Answers
Answer:
B
Explanation:
The appropriate diagram of the question is shown in the first image attached below.
From the diagram, we see the reaction of Cyclopentanol taking place under Tscl pyridine. We are to show the reaction mechanism and determine from the options, which appropriate product fits in.
So, from the reaction, the hydroxyl substituent reacts with Tscl where cl is being lost. This process is followed by an attack of N substituent on the pyridine with the Hydrogen atom and cleaves off for the structure to form a stable structure. The stereochemistry of the compound remains unchanged and it maintains its stick formula.
Thus, X is the appropriate and the correct product.
how to make 100 ml of 0.001 mM solution with 0.0405mM solution?
Answers
Answer:
Measure 2.47 mL of the stock solution (i.e 0.0405 mM) and dilute it to the 100 mL mark with water
Explanation:
To make 100 mL of 0.001 mM solution from 0.0405mM solution, we need to determine the volume of 0.0405mM solution needed. This can be obtained as follow:
Molarity of stock (M₁) = 0.0405 mM
Volume of diluted (V₂) = 100 mL
Molarity of diluted solution (M₂) = 0.001 mM
Volume of stock solution needed (V₁) =?
M₁V₁ = M₂V₂
0.0405 × V₁ = 0.001 × 100
0.0405 × V₁ = 0.1
Divide both side by 0.0405
V₁ = 0.1 / 0.0405
V₁ = 2.47 mL
Therefore, to make 100 mL of 0.001 mM solution from 0.0405mM solution, measure 2.47 mL of the stock solution (i.e 0.0405 mM) and dilute it to the 100 mL mark with water.
Students are asked to design an experiment with cookies to demonstrate their understanding of the scientific method. One group has decided to determine the amount of time it will take for a cookie to "dissolve" in a glass of milk.
Which variable would most likely NOT affect the time it takes for the cookie to dissolve?
a) the size of the cup of milk
b) the type of cookie
c) the number of students in the group
d) the temperature of the milk
e) the type of milk
Answers
I'm gonna guess E on this one, but I think you should choose either E or A
A cylinder of gas with a pressure of 8.0 atm is heated from 300. K to 600. K. What will the new pressure of the gas be?
Answers
Answer:
[tex]P_2=16atm[/tex]
Explanation:
Hello there!
In this case, according to the given information, we can infer we need to use the Gay-Lussac's equation it order to understand this pressure-temperature relationship as shown below:
[tex]\frac{P_1}{V_1}=\frac{P_2}{V_2}[/tex]
Thus, we solve for P2 and plug in P1, T1 and T2, to obtain:
[tex]P_2=\frac{P_1T_2}{T_1}\\\\P_2=\frac{8atm*600K}{300K}\\\\P_2=16atm[/tex]
Regards!
The lattice-like structure of a metal consists of negative metal ions in a "sea" of electrons.
O True
O False
Answers
Answer:
True
Explanation:
describe and compare the trends in hardness for group 1 and group 2
elements?
Answers
Answer:
The key difference between group 1 and group 2 elements is that all group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
The hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
What are the group 1 and 2 elements?The group 1 elements are alkali metals and group 2 elements are alkaline earth metals. Group 1 contain 7 elements. Group 2 has 6 elements.
The difference between, group 1 elements have unpaired electrons in their outermost orbital, whereas group 2 elements have paired electrons in their outermost orbital.
Thus, the hardness of group 2 elements are harder than group 1 elements because they have a strong metallic bond and atoms are closely packed.
Learn more about group 1 and 2 elements
https://brainly.com/question/867686
#SPJ2
If you have a solution that is 15 percent by mass of KCl in benzene, what is the new boiling point?
Answers
Answer:
https://www.chegg.com/homework-help/questions-and-answers/1-10-points-solution-15-percent-mass-kcl-benzene-new-boiling-point--901-c-b-921-c-c-821-c--q63751186
Explanation: Thats your answer
How many valance electrons does He need to get to 8
Answers
Answer:
Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons
Use the nutrition label to answer the following questions.
16.How much energy is contained in
the six-cookie serving size
recommended on the label?
17.How much energy in joules is provided by eating
six cookies? (1 cal = 4.184 J; ALSO: 1 Calorie = 1
kilocalorie)
Answers
Answer:
six cookies?(1 cal _4.184 j; ALSO: 1 Calorie -1 kilocalorie)
Use the scenario to answer the following question. A group of students working in a chemistry lab are planning a procedure to neutralize 10.0mL of 5.0 M hydrochloric acid (strong acid) with 5.0 M potassium hydroxide (strong base). In their procedure they plan on adding an equal volume of the base to the acid. What would be the expected outcome of carrying out this step as planned?
a- The resulting pH will be less than 7 because potassium hydroxide is less concentrated than the hydrochloric acid.
b- The resulting pH will not be able to be determined because the concentrations of the acid and base are not the same.
c- The resulting pH will be greater than 7 because potassium hydroxide is less concentrated than the hydrochloric acid.
d- The resulting pH will be equal to 7 because a strong base will neutralize a strong acid.
Answers
Answer:
d- The resulting pH will be equal to 7 because a strong base will neutralize a strong acid.
Explanation:
The reaction between potassium hydroxide and hydrochloric acid of equal volume and equal concentration yields a solution of pH 7 at equivalence point. We must note that KOH is a strong base while HCl is a strong acid. This fact influences the pH of the system at equivalence point.
Owing to the fact that the acid is exactly neutralized by the base; at the equivalence point of such titration, it is expected that hydrogen ions(H+) and hydroxide ions (OH-) must have reacted to form water, this leads to a final pH of 7.
Can someone give me an example of balancing equations with a solution that is simple?
Answers
Explanation:
[tex]H _{2}O _{2(aq)} →H _{2}O _{(l)} + O _{2}(g) \\ solution : 2 \: and\: 2[/tex]
The equation:
K + HOH -> KOH + H2 (an example of a single replacement reaction)
Balanced equation:
2 K + 2 HOH -> 2 KOH + 1 H2
In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) H2O (g) CO2 (g) H2 (g) In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is __________. A) 5.47 B) 1.0 C) 1.78 D) 0.75 E) 0.56
Answers
Answer: [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
Explanation:
Moles of [tex]CO[/tex] = 0.35 mole
Moles of [tex]H_2O[/tex] = 0.40 mole
Volume of solution = 1.00 L
Initial concentration of [tex]CO[/tex] = [tex]\frac{0.35mol}{1.00L}=0.35M[/tex]
Initial concentration of [tex]H_2O[/tex] = [tex]\frac{0.40mol}{1.00L}=0.40M[/tex]
Equilibrium concentration of [tex]CO[/tex] = [tex]\frac{0.19mol}{1.00L}=0.19M[/tex]
The given balanced equilibrium reaction is,
[tex]CO(g)+H_2O(g)\rightleftharpoons CO_2(g)+H_2(g)[/tex]
Initial conc. 0.35 M 0.40 M 0 M 0M
At eqm. conc. (0.35-x) M (0.40-x) M (x) M (x) M
Given: (0.35-x) = 0.19
x= 0.16 M
The expression for equilibrium constant for this reaction will be,
[tex]K_{eq}=\frac{[CO_2]\times [H_2]}{[CO]\times [H_2O]}[/tex]
Now put all the given values in this expression, we get :
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.35-0.16)\times (0.40-0.16)}[/tex]
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.19)\times (0.24)}=0.56[/tex]
Thus [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
The tertiary structure of a protein is a complex arrangement formed as the polypeptide chain folds and twists.
a. True
b. False
Answers
Answer:
True
Explanation:
The tertiary structure of a protein is a complex arrangement formed as the polypeptide chain folds and twists.
This folding & twisting of polypeptide chain leading to its complex structure, is true about tertiary structure of protein. It occurs due to different interactions between side chains of amino acids.
pls help Which statement is true about the environment of urban areas?
Urban areas have higher temperatures.
Urban areas have few problems with soil erosion.
Urban areas have more rain infiltration into the soil.
Urban areas have less habitat fragmentation.
Answers
The statement that is correct about Urban areas is that they have higher temperatures.
Urban Area is a term to refer to cities. Urban areas are characterized by having a developed infrastructure (wide roads, vehicular bridges, wide platforms, tall buildings, residential areas, industrial areas, among others).
Recent studies affirm that urban areas are warmer than surrounding areas; This phenomenon is because the materials with are built buildings, roads, houses, and others, concentrate the sun's rays, increasing the temperature of cities. In addition, the lack of trees deepens this phenomenon, because the trees contribute to cooling by physicochemical processes such as evapotranspiration.
According to the above, it is possible to affirm that urban areas are hotter than their surrounding area because they lack vegetation, and the materials with which it is built contribute to the increase in temperature.
On the other hand, urban areas are characterized by habitat fragmentation, more problems with soil erosion, and less rain infiltration into the soil.
Learn more in: https://brainly.com/question/23587978
Answer:
A) higher temps
Explanation:
what is the major organic product obtained from the following sequence of reactions 1. naoch2ch3 ch3ch2oh 2. ph br 1. naoch2ch3 ch3ch2oh 2. br 1. lialh4 et2o 2. h3o
Answers
______ is the process of change from a liquid to a gas at temperatures below the boiling
point.
Answers
Answer:
evaporation is the process
Answer:
EvaporationExplanation:
Evaporation is the process of becoming a vapor. The process of extracting moisture as by heat.
Evaporation is the process of change from a liquid to a gas at temperatures below the boiling point.
Therefore, the final answer is evaporation.
Select the correct answer.
Using this activity chart, which reaction will happen when a piece of copper is placed in a lead nitrate solution?
A.
2Cu + 3Pb(NO3)2 3Cu(NO3)2 + 2Pb
B.
No reaction occurs.
C.
2Cu + 3Pb(NO3)2 2Cu(NO3)2 + 3Pb
D.
3Cu + 3Pb(NO3)2 3Cu(NO3)2 + 3Pb
E.
The answer cannot be determined from the information given.
Answers
Answer:
B, No reaction will occur
Explanation:
Copper as compared to lead is less reactive. This is the reason when lead is added to copper nitrate solution, it replaces the copper and itself combines with nitrate to form lead nitrate aqueous solution
Lead + Copper(II) nitrate → Copper + Lead (II) nitrate
The same is not the case when the reaction is revered i.e Cu is added to Pb NO3 solution.
Hence, option B is correct
What is the name of Na
Answers
mdmsjdkskdkskdjxjxjjz
If a particular ore contains 56.3 % % calcium phosphate, what minimum mass of the ore must be processed to obtain 1.00 kg k g of phosphorus?
Answers
Answer:
34.44 kg
Explanation:
First we convert 1.00 kg of phosphorus (P) into moles, using its molar mass:
1.00 kg ÷ 32 kg/kmol = 0.03125 kmol PThen we convert 0.03125 kmoles of P into kmoles of Ca₃(PO₄)₂:
0.03125 kmol P * [tex]\frac{2kmolCa_3(PO_4)_2}{2kmolP}[/tex] = 0.0625 kmol Ca₃(PO₄)₂Now we calculate the mass of 0.0625 kmoles of Ca₃(PO₄)₂:
0.0625 kmol Ca₃(PO₄)₂ * 310.18 kg/kmol = 19.39 kgFinally we calculate the required mass of the ore, using the definition of content percentage:
% content = Mass of calcium phosphate / mass of ore * 100 %56.3 % = 19.39 kg / mass of ore * 100%Mass of Ore = 34.44 kgAll of the following are advantages of nuclear power EXCEPT: uranium mines cause less environmental damage than coal mines because less uranium is needed to generate power. nuclear power plants generate no nitrogen oxides and sulfur dioxide. uranium generates far greater amounts of energy than coal by weight or volume. the power-generating process is emission-free. nuclear wastes can be safely disposed of.
Answers
Answer:
nuclear wastes can be safely disposed of
Explanation:
In comparing fossil fuel power plants with nuclear power plants, it is obvious that fossil fuel power plants lead to a large volume of emission of oxides of carbon and sulphur.
Also, a larger volume of coal needs to be burnt to generate energy compared to a minute amount of uranium fuel that can sustain a nuclear power plant for a long period of time thereby reducing the environmental damage associated with mining of the fuel.
However, the problem of nuclear waste disposal have remained a thorn in the flesh. It is often difficult to safely dispose of spent uranium fuel. This is a major disadvantage of the use of nuclear power.
bakit mahalaga ang ilaw trapiko?
grade 3
Answers
Explanation:
#IHOPEIHELPYOU PA BRAINLY NA LANG PO
Find the % composition for each element in Zinc Chlorate
Answers
Answer:
chlorine ~ 30%
zinc ~ 28%
oxygen ~ 41%
Explanation:
Hydrogen iodide can decompose into hydrogen and iodine gases. 2HI(g) H2(g) I2(g) K for the reaction is 0.016. Of 0.148 atm of HI(g) is sealed in a flask, what is the pressure of each gas when equilibrium is established
Answers
Solution :
Given :
Hydrogen iodide decomposes to hydrogen and iodine gas
[tex]$2 HI \ \ \ \Leftrightarrow \ \ \ \ H_2 \ \ \\ + \ \ I_2 $[/tex]
I 0.148 0 0
C -2a +a +a
E 0.148-2a a a
We know
[tex]$k_p=\frac{P(H_2)P(I_2)}{P(HI)^2}$[/tex]
[tex]$0.016=\frac{a^2}{(0.148-2a)^2}$[/tex]
[tex]$0.016^{1/2}=\frac{a}{0.148-2a}$[/tex]
[tex]$0.12649=\frac{a}{0.148-2a}$[/tex]
0.0187 = 1.2529 a
a = 0.0149
Therefore
P(HI) = 0.148 - 2a
= 0.148 - 2(0.0149)
= 0.1182 atm
P([tex]$H_2$[/tex]) = a
= 0.0149 atm
P([tex]$I_2$[/tex]) = a
= 0.0149 atm
What type of reaction is illustrated?
C3H8 +502 + 3C02 + 4H2O
А
B
decomposition
combustion
single
replacement
Answers
Answer:
Combustion
Explanation:
A sample of air was collected on a day when the total atmosphere
pressure was 592 mmHg. The sample contained only oxygen and
nitrogen gas. If the oxygen in the sample had a pressure of 261
mmHg, how much pressure did the nitrogen have?
A. 853 mm Hg
B. 0.206 mm Hg
C. 4.76 mm Hg
D. 331 mm Hg
E. Other________
(Please show me how you did it)
Answers
Answer:
D. 331 mm Hg
Explanation:
We can solve this problem by keeping in mind the law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of its components.
In other words:
P oxygen + P nitrogen = Total PWe input the given data:
261 mmHg + P nitrogen = 592 mmHgAnd calculate the pressure of the nitrogen:
P nitrogen = 331 mmHgCalculate the freezing point of a solution made by dissolving 13 g potassium sulfide in 150 g H2O Kf for water = 1.86 C/m
Answers
Answer:
[tex]T_F=-4.4\°C[/tex]
Explanation:
Hello there!
In this case, according to the given information, it is possible for us to calculate the freezing point of an aqueous solution of potassium sulfide by using the following equation:
[tex]T_F=T_{solv}-i*m*Kf[/tex]
In such a way, we firstly calculate the molality of this solution according to:
[tex]m=\frac{\frac{13g}{110.262 g/mol} }{0.150kg} =0.79m[/tex]
Finally, since the Van't Hoff's factor for K2S is 3, the freezing point of the solution turns out to be:
[tex]T_F=0\°C-3*0.79m*1.86\°C/m\\\\T_F=-4.4\°C[/tex]
Regards!
During a combustion reaction, 9.00 grams of oxygen reacted with 3.00 grams of CH4.
What is the amount of the leftover reactant?
0.74 grams of methane
0.89 grams of methane
1.22 grams of oxygen
1.45 grams of oxygen
Answers
Answer:d
Explanation: