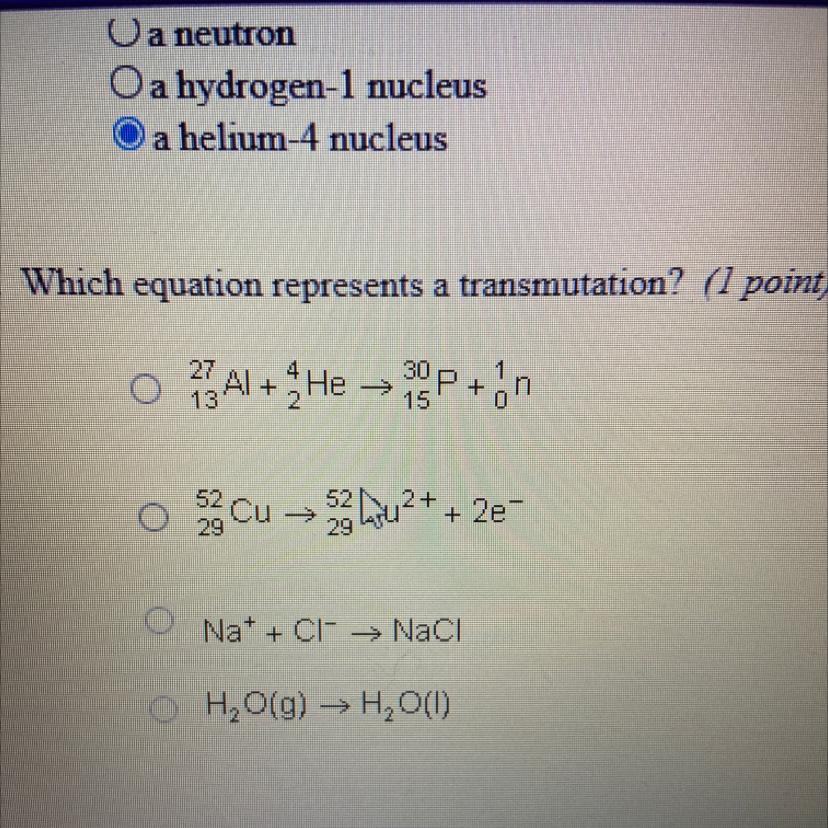
Answers
Answer:
A
Explanation:
edge21
Related Questions
During a combustion reaction, 9.00 grams of oxygen reacted with 3.00 grams of CH4.
What is the amount of the leftover reactant?
0.74 grams of methane
0.89 grams of methane
1.22 grams of oxygen
1.45 grams of oxygen
Answers
Answer:d
Explanation:
7. What is the mass of a piece of copper (Cu) that undergoes a 25.0 °C
temperature change with the loss of 428 J of energy?
Answers
Answer:
0.0428 kg or 42.8 g
Explanation:
Applying,
Q = cmΔt.............. Equation 1
Where Q = heat lost of heat gained, c = specific heat capacity of copper, m = mass of copper, Δt = temperature change.
make m the subject of the equation above
m = Q/cΔt.................. Equation 2
From the question,
Given: Q = 428 J, Δt = 25 °C
Constant: c = 400 J/kg.°C
Substitute these values into equation 2
m = 428/(25×400)
m = 0.0428 kg
m = 42.8 g
QA49 (N13/13/Q9)
Use of the Data Booklet is relevant to this question.
When an evacuated fluorescent light tube of volume 300 cm^3 is filled with a gas at 300K and
101 kPa, the mass of the tube increases by 1.02g. The gas obeys the ideal gas equation
What is the identity of the gas?
A argon
B krypton
C neon
D nitrogen
Answers
Students are asked to design an experiment with cookies to demonstrate their understanding of the scientific method. One group has decided to determine the amount of time it will take for a cookie to "dissolve" in a glass of milk.
Which variable would most likely NOT affect the time it takes for the cookie to dissolve?
a) the size of the cup of milk
b) the type of cookie
c) the number of students in the group
d) the temperature of the milk
e) the type of milk
Answers
I'm gonna guess E on this one, but I think you should choose either E or A
A solution with a pH of 5.30 has a H+ concentration of
Answers
Answer:
5.01 x 10^-6 M
Explanation:
PH= -log [H+]
[H+] = 10^-PH
In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) H2O (g) CO2 (g) H2 (g) In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is __________. A) 5.47 B) 1.0 C) 1.78 D) 0.75 E) 0.56
Answers
Answer: [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
Explanation:
Moles of [tex]CO[/tex] = 0.35 mole
Moles of [tex]H_2O[/tex] = 0.40 mole
Volume of solution = 1.00 L
Initial concentration of [tex]CO[/tex] = [tex]\frac{0.35mol}{1.00L}=0.35M[/tex]
Initial concentration of [tex]H_2O[/tex] = [tex]\frac{0.40mol}{1.00L}=0.40M[/tex]
Equilibrium concentration of [tex]CO[/tex] = [tex]\frac{0.19mol}{1.00L}=0.19M[/tex]
The given balanced equilibrium reaction is,
[tex]CO(g)+H_2O(g)\rightleftharpoons CO_2(g)+H_2(g)[/tex]
Initial conc. 0.35 M 0.40 M 0 M 0M
At eqm. conc. (0.35-x) M (0.40-x) M (x) M (x) M
Given: (0.35-x) = 0.19
x= 0.16 M
The expression for equilibrium constant for this reaction will be,
[tex]K_{eq}=\frac{[CO_2]\times [H_2]}{[CO]\times [H_2O]}[/tex]
Now put all the given values in this expression, we get :
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.35-0.16)\times (0.40-0.16)}[/tex]
[tex]K_{eq}=\frac{0.16\times 0.16}{(0.19)\times (0.24)}=0.56[/tex]
Thus [tex]K_{eq}[/tex] at the temperature of the experiment is 0.56.
The lattice-like structure of a metal consists of negative metal ions in a "sea" of electrons.
O True
O False
Answers
Answer:
True
Explanation:
A tau lepton decays into an electron, an electron antineutrino and a tau neutrino. Write out this reaction in symbolic (equation) form and show that charge and lepton number is conserved. 2. What is the total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons
Answers
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
The total number of quarks in a helium nucleus consisting of 2 protons and 2 neutrons is 12 quarks.
What is the meaning of lepton?Any of a family of particles (such as electrons, muons, and neutrinos) that have spin quantum number ¹/₂ and that experience no strong forces.
The equation in the symbolic form can be written as,
[tex]\tau \;- > e- + {\bar{\nu }} e + \nu\tau[/tex]
Charge on the left side = -1
Charge on the right side = -1+0+0 = -1
As the charge on the electron antineutrino, [tex]{\bar{\nu }}[/tex] e and the tau neutrino, [tex]\nu\tau[/tex] are zero each.
Thus, charge on left side = charge on right side
Or, the equation is in accordance with charge conservation.
Lapton number of left side = 1
Laptop number of right side = 1-1+1 = 1
This is because, lapton number of the particles involved are,
Tau lapton, [tex]\tau[/tex]-1 = 1,
Electron, e-1 = 1,
Electron antineutrino, [tex]{\bar{\nu }}[/tex]e = -1,
Tau neutrino, [tex]\nu\tau[/tex]= 1,
Thus, lapton number of left side = lapton number of right side
So, the equation obeys lapton number conservation.
2.
The total number quarks in a helium nucleus which is composed of 2 protons (positively charged particle) and 2 neutrons (uncharged particle) is:
Since there 3 quarks in each nucleon, 4 nucleons would have a total of 12 quarks.
Just simply multiply 3 quarks by the number of nucleons:
So, 3 (quarks) x 4 (nucleons) = 12 quarks
Therefore, there are 12 quarks in 4 nucleons.
Learn more about lepton decays here:
https://brainly.com/question/12044097
#SPJ1
(NH4)2Cr2O7 Cr2O3 + N2 + H2O
If 4.7369 moles of H2O are produced, how many moles of (NH4)2Cr2O7 were reacted?
Answers
Answer:
the original substances in any chemical reaction. products. the resulting substances in any....chromium(III) oxide, and water. (NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) + 4H2O(g).
How to solve for K when given your anode and cathode equations and voltage
Answers
Answer:
See Explanation
Explanation:
In thermodynamics theory the Free Energy (ΔG) of a chemical system is described by the expression ΔG = ΔG° + RTlnQ. When chemical system is at equilibrium ΔG = 0. Substituting into the system expression gives ...
0 = ΔG° + RTlnKc, which rearranges to ΔG° = - RTlnKc. ΔG° in electrochemical terms gives ΔG° = - nFE°, where n = charge transfer, F = Faraday Constant = 96,500 amp·sec and E° = Standard Reduction Potential of the electrochemical system of interest.
Substituting into the ΔG° expression above gives
-nFE°(cell) = -RTlnKc => E°(cell) = (-RT/-nF)lnKc = (2.303·R·T/n·F)logKc
=> E°(cell) = (0.0592/n)logKc = E°(Reduction) - E°(Oxidation)
Application example:
Calculate the Kc value for a Zinc/Copper electrochemical cell.
Zn° => Zn⁺² + 2e⁻ ; E°(Zn) = -0.76 volt
Cu° => Cu⁺² + 2e⁻ ; E°(Cu) = 0.34 volt
By natural process, charge transfer occurs from the more negative reduction potential to the more positive reduction potential.
That is,
Zn° => Zn⁺² + 2e⁻ (Oxidation Rxn)
Cu⁺² + 2e⁻ => Cu° (Reduction Rxn)
E°(Zn/Cu) = (0.0592/n)logKc
= (0.0592/2)logKc = E°(Cu) - E°(Zn) = 0.34v - (-0.76v) = 1.10v
=> logKc = 2(1.10)/0.0592 = 37.2
=> Kc = 10³⁷°² = 1.45 x 10³⁷
PLZ ANSWER QUICK I AM IN TIMER HELP WILL GIVE BRAINLIEST
Which is greater, the moon's period of rotation or its period of revolution?
They are equal.
the moon's revolution period around Earth
Neither are known.
the moon's rotational period
Answers
Answer:
B
Explanation:
Question 4 (10 points)
If a sollition has a pOH of 5.2 the [OH-] of the solution is
оа
6x 10-6 M
Ob
6.3 x 10-6 M
Oc
1.58 x 10-5M
Od
2x10-5M
Answers
Answer:
Explanation:
Which statement describes the atoms in an element
Answers
Answer:
Atoms are kind of like building blocks.
Explanation:
Think of atoms as kind of like a mix between paint pigments and legos. The more legos that you have, the bigger the molecule but keep in mind that there are many different kinds of legos that all can come together to build numerous things. I hope that I answered your question and i apoligize if I didn't.
PLEASE HELP, DUE AT 12:00
Answers
How many moles of nitrogen are required to produce 13.5 g of NH 3?
Answers
Answer:
number of moles of (N) = 0.794 moles
Explanation:
From the given information:
no of moles of nitrogen (N) = (unknown)???
mass of nitrogen = 13.5 g
molar mass of NH3 = 14 +( 1 × 3) = 17 g/mol
To calculate the no of moles of N, we have:
number of moles of (N) = mass of N/molar mass
number of moles of (N) = 13.5 g/17 g/mol
number of moles of (N) = 0.794 moles
Using Reaction A, how many grams of CO2 can be created from 5.67 moles of water?
127.6 g CO2
199.6 g CO2
81.65 g CO2
311.85 g CO2
Answers
Answer:
81.65 g CO2
Explanation:
14. Simply, explain the role of both the nucleus and the ribosome in protein synthesis.
Answers
Answer:
Eukaryotic cells have a true nucleus, which means the cell's DNA is surrounded by a membrane. Therefore, the nucleus houses the cell's DNA and directs the synthesis of proteins and ribosomes, the cellular organelles responsible for protein synthesis.
Ribosomes are the sites in a cell in which protein synthesis takes place. ... Within the ribosome, the rRNA molecules direct the catalytic steps of protein synthesis — the stitching together of amino acids to make a protein molecule.
How does carbonic acid work to maintain blood pH? (Select all that apply.) Check All That Apply When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid is added to the blood until the pH reaches 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid always lowers the blood pH to 7.4. Carbonic acid can raise or lower the pH of blood. Carbonic acid can raise or lower the pH of blood.
Answers
Answer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Carbonic acid can raise or lower the pH of blood.
Explanation:
A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. The human blood serves as a buffer as it contains a buffer of carbonic acid (H2CO3) and bicarbonate anion (HCO3-) which serves to maintain blood pH between 7.35 and 7.45. Other buffering systems in blood exist such as the Hydrogen ion and oxygen gas which affects oxygen binding to haemoglobin, however the carbonic-acid-bicarbonate buffer is the most important buffer for maintaining acid-base balance in the blood.
A buffer solution is made up of an acid and its conjugate base or a base and its conjugate acid. For carbonic acid-bicarbonate buffer, carbonic acid serves as the acid while bicarbonate serves as the base. When a little quantity of a base as hydroxide ions is added to a buffer, the acid reacts with it and remove it from the solution. On the other hand, when a little quantity of an acid as hydrogen ions are added to a buffer, the conjugate base reacts with it and remove it from the solution, thus keeping the pH of the solution fairly constant.
In the carbonic acid-bicarbonate buffer:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.
When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.
Thus, carbonic acid can raise or lower the pH of blood.
Carbonic acid work to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.WHAT IS BUFFER SOLUTION:A buffer is a solution that resists changes to its pH when small quantities of acids or bases are added to it. A buffer is made up of an acid and its conjugate base or a base and its conjugate acid. Carbonic acid is an example of buffer that contains an acid with it's conjugate base.This means that, carbonic acid works to maintain blood pH as follows:
When blood is too basic, carbonic acid can ionize to bicarbonate and H+ ions, adding H+ ions to the blood.When blood becomes too acidic, bicarbonate combines with extra H+ ions to form carbonic acid, removing H+ ions from the blood.Carbonic acid can raise or lower the pH of blood.Learn more about buffers at: https://brainly.com/question/24188850
Select the correct answer.
Using this activity chart, which reaction will happen when a piece of copper is placed in a lead nitrate solution?
A.
2Cu + 3Pb(NO3)2 3Cu(NO3)2 + 2Pb
B.
No reaction occurs.
C.
2Cu + 3Pb(NO3)2 2Cu(NO3)2 + 3Pb
D.
3Cu + 3Pb(NO3)2 3Cu(NO3)2 + 3Pb
E.
The answer cannot be determined from the information given.
Answers
Answer:
B, No reaction will occur
Explanation:
Copper as compared to lead is less reactive. This is the reason when lead is added to copper nitrate solution, it replaces the copper and itself combines with nitrate to form lead nitrate aqueous solution
Lead + Copper(II) nitrate → Copper + Lead (II) nitrate
The same is not the case when the reaction is revered i.e Cu is added to Pb NO3 solution.
Hence, option B is correct
convert 113 Fahrenheit to celcius do full process
Answers
[tex]\huge{\textbf{\textsf{{\color{pink}{An}}{\red{sw}}{\orange{er}} {\color{yellow}{:}}}}}[/tex]
45° Celsius
Formula (113°F − 32) × 5/9 = 45°C
ThanksHope it helpsThere are 6 different organisms in the picture above. Organize them into 2 or more groups AND defend your groups by
providing details about why you put them into the chosen groups.
Example on how to answer:
Group 1- XYZ
Group 2-LM
Group 3- PORS
I put XYZ in group 1 because...
I put LM together because...
I grouped PORS together because...
Answers
Explanation: they could be grouped by how they reproduce or they can be grouped by if there hetero or autotrophic
Which of the following is an example of an environmental impact of agriculture?
high use of mineral resources
high use of water
high use of gold, copper, and silver
high use of rock supplies
Answers
Answer:
high use of mineral resources
Explanation:
brainliest pls
Which of the following particles have the same mass. Proton, Neutron, Electron, None
Answers
Answer: proton and neutron
Explanation:
They both have the mass of 1
What is the name of Na
Answers
mdmsjdkskdkskdjxjxjjz
Can you tell me any chemical reaction that occur due to kinetic energy
Answers
Answer:
The molecules in gasoline (octane, the chemical formula shown) contain chemical energy. This energy is transformed into kinetic energy that allows a car to race on a racetrack.
write a story of your life when you were hurted by someone whom you trusted blindly...
Answers
Answer:
Sis I think it happened with me but I am not able to remember if u want u can share if it happened with u
Que uso le darías a la vida diaria escribe en español plis
Answers
Explanation:
Facilitar el movimiento y desplazamiento de objetos pesados reduciendo el rose del objeto contra el suelo, Obtener un movimiento circular por efecto de la fuerza de un liquido, en el caso de contadores, molinos, centrales hidroeléctricas y turbinas.
Transmitir el movimiento de un eje a otro como en el caso de las lavadoras, bicicletas y neveras. Reducir drásticamente el esfuerzo necesario para elevar y mover objetos, como en casos de pozos de agua y ascensores Transformar movimientos giratorios en otros movimientos o viceversa. ,
explain the process of finishing
Answers
Answer:
[Your answer would be in this document of mine below] Thanks :D
Explanation:
A sample of air was collected on a day when the total atmosphere
pressure was 592 mmHg. The sample contained only oxygen and
nitrogen gas. If the oxygen in the sample had a pressure of 261
mmHg, how much pressure did the nitrogen have?
A. 853 mm Hg
B. 0.206 mm Hg
C. 4.76 mm Hg
D. 331 mm Hg
E. Other________
(Please show me how you did it)
Answers
Answer:
D. 331 mm Hg
Explanation:
We can solve this problem by keeping in mind the law of partial pressures, which states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of its components.
In other words:
P oxygen + P nitrogen = Total PWe input the given data:
261 mmHg + P nitrogen = 592 mmHgAnd calculate the pressure of the nitrogen:
P nitrogen = 331 mmHgThe ionization potential of Be atom is more than expected because it has -
(a) half filled valence p orbitals
(b) fully filled valence s orbitals
(c) both a and b
Answers
Answer:
(b) fully filled valence s orbitals
Explanation:
Electron configuration of Be: 1s22s2
2s2 is fully filled