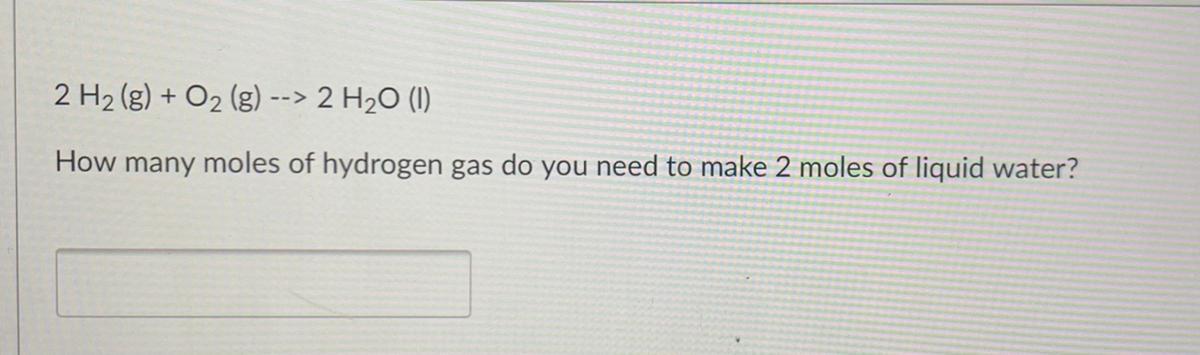
2 H2(g) + O2 (g) --> 2 H20 (1)
How many moles of hydrogen gas do you need to make 2 moles of liquid water?
Answers
Answer:
4moles of hydrogen atoms
Explanation:
The second conversion factor reflects the number of atoms contained within each molecule. Two water molecules contain 4 hydrogen atoms and 2 oxygen atoms. A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms.
Related Questions
What is an example of Chemical energy to thermal energy to electrical current?
Answers
Answer:
Coal is burned at power plant.
Explanation:
the chemical Energy is released as coal changes
have a good day
Biodiversity can be altered by __________.
Select one:
All of these
a fire in the ecosystem
unexpected changes in the climate of an ecosystem
a flood in the ecosystem
Answers
How many moles of gallium chlorate, Ga(ClO3)3, must react in order to produce 674 kJ of energy according to the following reaction? 2 Ga(ClO3)3 à 2 GaCl3 + 9 O2 DH = - 130.4 kJ
Answers
Answer:
10.3 mol Ga(ClO₃)₃
Explanation:
Let's consider the following balanced thermochemical equation.
2 Ga(ClO₃)₃(s) ⇒ 2 GaCl₃(s) + 9 O₂(s) ΔH = - 130.4 kJ
According to the balanced thermochemical equation, 130.4 kJ of heat are released when 2 moles of gallium chlorate react. The number of moles of gallium chlorate that must react to produce 674 kJ of energy is:
674 kJ × 2 mol Ga(ClO₃)₃/130.4 kJ = 10.3 mol Ga(ClO₃)₃
Thermochemical equations are chemically balanced equations that take into account both the energy change and the physical states of all reactants and products. Energy is a reactant in an endothermic process, whereas it is a product in an exothermic reaction. Here the moles of Ga(ClO₃)₃ is 10.3 mol.
Let's consider the following balanced thermochemical equation.
2Ga(ClO₃)₃(s) ⇒ 2GaCl₃(s) + 9O₂(s) ΔH = - 130.4 kJ
According to the balanced thermochemical equation, 130.4 kJ of heat is released when 2 moles of gallium chlorate react. The number of moles of gallium chlorate that must react to produce 674 kJ of energy is:
674 kJ × 2 mol Ga(ClO₃)₃ / 130.4 kJ = 10.3 mol Ga(ClO₃)₃
To know more about thermochemical equation, visit;
https://brainly.com/question/10384873
#SPJ6
10OIS
Save for Later
1
Yusef is doing an experiment to find out how the amount of sunlight affects the growth of a seedling. He has three identical pots
containing grass seeds he planted at the same time. He puts them in windows that receive different amounts of sunlight each day.
Every day. Yusef looks at the pots and records his observations. What is the independent variable in Yusef's experiment?
OA type of soil
OB type of seedling
Ос.
amount of water
OD
amount of sunlight
Reset
Next Question
Answers
Answer:
asdffafAFSAfdsdf
Explanation:
Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. A mixture of 41.0 g of magnesium and 175.0 g of iron(III) chloride is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete. Select one: a. Limiting reactant is Mg; 7.4 g of FeCl3 remain. b. Limiting reactant is Mg; 46.5 g of FeCl3 remain. c. Limiting reactant is FeCl3; 1.7 g of Mg remain. d. Limiting reactant is FeCl3; 37.8 g of Mg remain. e. Limiting reactant is Mg; 134.0 g of FeCl3 remain.
Answers
Answer:
Limiting reactant is FeCl3; 1.7 g of Mg remain.
Explanation:
From the question;
The equation is;
3Mg(s) + 2FeCl₃(s) → 3MgCl₂(s) + 2Fe(s)
Amount of Mg = 41 g/24.31 g/mol = 1.687 moles
The limiting reactant yields the least amount of MgCl2
3 moles of Mg yields 3 moles of MgCl2
Hence 1.687 moles of Mg yields yields 1.687 moles of MgCl2
FeCl₃ = 175 g/162.2 g/mol = 1.0789 moles
2 moles of FeCl3 yields 3 moles of MgCl2
1.0789 moles of FeCl3 yields 1.0789 * 3/2 = 1.61835 moles of MgCl2
Hence FeCl3 is the limiting reactant
3 moles of Mg reacts with 2 moles of FeCl3
x moles of Mg reacts with 1.0789 of FeCl3
x = 3 * 1.0789 /2 = 1.61835 moles of Mg
Mass of Mg reacted = 1.61835 moles * 24.31 = 39.342 g
Mass of excess Mg = 41 g - 39.342 g = 1.7 g
describe how to calculate the relative atomic mass
Answers
Here is your answer!!
You can always find the relative mass of an element by adding the number of protons to the number of neutrons for the specific isotope of the element you're considering. For example, a carbon-12 atom has 6 protons and 6 neutrons, and so has a relative atomic mass of 12.
Hope this helps please consider giving brainliest!!! Have a good day!!<3
The tetrahedral arrangement of bonds formed by carbon and its relevance In the interaction of biomolecules
Answers
Answer:
Due to the presence of 4 valence electrons.
Explanation:
The tetrahedral arrangement of bonds formed by carbon and its relevance In the interaction of biomolecules occurs because the carbon atom has four electrons in their outermost shell and the four hydrogens has one electron each which result is a total of eight electrons distributed in four bonding orbitals making the structure of tetrahedral and the bond angles between these carbon atoms is approximately 109.5 degree.
If 36g of Al react with 34L of Oz, how many moles of Al2O3 are produced?
4Al +302 --> 2Al2O3
145 moles
0.667 moles
0.165 moles
101 moles
Answers
Answer:
.........................................................................................................................................................................................................................................................................................................................................................................................................................................................
Explanation:
If 36g of Al react with 34L of Oz, 0.667 moles of Al2O3 are produced. The correct option is b, 0.667 moles
What are moles?The mole is a SI unit of measurement that is used to calculate the quantity of any substance.
[tex]\rm 4Al +3O_2 -- > 2Al_2O_3[/tex]
Step1- calculate the moles of aluminum
The mass of Al is 36 g
The molar mass of Al is 27
[tex]\rm Number\;of \;moles= \dfrac{mass}{molar\;mass}\\\rm Number\;of \;moles\;of\;Al = \dfrac{36}{27} = 1.33\;mol[/tex]
Step2- calculate the moles of oxygen
The mass of O₂ is 34 L
[tex]\rm 1 L = \dfrac{1}{22.4 }[/tex]
[tex]\rm Number\;of \;moles\;O_2= \dfrac{34}{22.4} = 1.51[/tex]
The limiting reagent is 1.33 MOL. By the rule of stoichiometry, four moles of Al produced 2 moles of Al.
[tex]\rm \dfrac{1.33}{2} = 0.665 \;moles[/tex]
Thus, the correct option is b. 0.667 moles.
Learn more about moles
https://brainly.com/question/26416088
#SPJ2
Which of the following is most helpful for identifying an organism using a dichotomous key?
Its average lifespan
Its sources of food
Its habitat
Its physical structures
Answers
Answer:
Its physical structure
Explanation:
hahaha
ok fine
stream fever
engenes
Select the correct structure that
corresponds to the name.
4-bromo-5-chlorocyclohexene
Answers
Answer: a
Explanation:
Which statement describes the Richter scale?
It cannot account for fault movement during an earthquake.
It measures large earthquakes far from the seismograph.
It estimates the total energy released from an earthquake.
It increases in magnitude as amount of damage increases.
Answers
Answer:
It measures large earthquakes far from the seismograph.
Explanation:
It uses a seismograph to measure earthquakes far from the seismograph where seismic waves are used to determine the magnitude of an earthquake.
Answer: d. It increases in magnitude with an increase in size of seismic waves.
Explanation:
4. Use the particle theory to explain density.
Answers
Answer:
Density is the total number of particles in a given volume
Explanation:
As per the particle theory, density is defined as total space occupied by certain number of particles of any given element with in a defined volume is termed as density.
Density is not the denseness of a single unit but the space occupied by many single units with in a given volume V
Hence, density is the total number of particles in a given volume.
how many carbon atoms are in 26-hydrogen alkyne
Answers
A substance has a boiling point of 78 °C. Which of the following is true about the substance? (5 points) a It will also change from a gas to a solid at 78 °C while the gas loses energy. b It will also change from a gas to a solid at 78 °C while the gas gains energy. c It will also change from a gas to a liquid at 78 °C while the gas loses energy. d It will also change from a gas to a liquid at 78 °C while the gas gains energy.
Answers
Answer: If a substance has a boiling point of [tex]78^{o}C[/tex] then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
Explanation:
The temperature at which vapor pressure of a liquid substance becomes equal to the atmospheric pressure is called boiling point of substance.
At the boiling point, liquid phase and vapor phase remains in equilibrium.
This means that as liquid phase changes into vapor phase and also vapor phase changes into liquid phase at the boiling point.
Thus, we can conclude that if a substance has a boiling point of [tex]78^{o}C[/tex] then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
CuSO4 + 2NaBr --> CuBr2 + Na2SO4 ? How many grams of CuSO4 are needed to react with 1.80 x 1024 molecules of NaBr?
Answers
Answer:
[tex]238.5gCuSO_4[/tex]
Explanation:
Hello there!
In this case, by considering the given chemical reaction, it is possible for us to calculate the required grams of CuSO4 by considering its molar mass of 159.6 g/mol, the given molecules of NaBr, the Avogadro's number and the 1:2 mole ratio between these two in order to set up the following stoichiometric set up:
[tex]1.80x10^{24}moleculesNaBr*\frac{1molNaBr}{6.022x10^{23}molecules NaBr}*\frac{1molCuSO_4}{2molNaBr} *\frac{159.6gCuSO_4}{1molCuSO_4}\\\\=238.5gCuSO_4[/tex]
Best regards!
How many formula units (particles of AgNO3) are in 5.50
grams of AgNO3?
a 1.93 x 1022 molecules
b 8.67 x 1021 molecules
C 0.032 molecules
d 6.14 1022 molecules
e, 3.22 x 1022 molecules
Answers
Answer:
2.0 X 10²² molecules of AgNO₃Explanation:
Given 5.50 grams of AgNO₃, how many formula units of AgNO₃ are contained in the 5.50 grams of AgNO₃? (formula wt. of AgNO₃ = 169,87 g/mole).
Solution:
1st convert grams to moles => moles AgNO₃ = 5.50 g AgNO₃ / 169.841g AgNO₃ = 0.032377 mole AgNO₃ 2nd calculate number of particles in 0.032377 mole AgNO₃ . Number AgNO₃ molecules in 0.032377 mole AgNO₃ = 0.032377 mole AgNO₃ X 6.023 x 10²³ molecules of AgNO₃ /1.0 mole AgNO₃ = 1.95007 X 10²² molecules of AgNO₃ in 5.50 grams of AgNO₃. The appropriate form of the answer should contain 2 Sig,Figs. based on the data point having the least number of sig.figs. in the given data. This then is 5.50 grams of AgNO₃ which has 2 sig.figs. Therefore, the number of AgNO₃ molecules in 5.5 grams of AgNO₃ = 2.0 X 10²² molecules of AgNO₃. (Note: 1.95 rounds to 2.0).The number of molecules of silver nitrate in 5.5 grams has been [tex]1.93\;\times\;10^2^2[/tex]. Thus, option A is correct.
The formula unit has been given as the number of molecules of the compound. The number of molecules in a mole of compound has been given by the Avogadro law.
According to the Avogadro law, the number of molecules in a mole of sample has been equivalent to the Avogadro number, i.e. [tex]6.023\;\times\;10^2^3[/tex].
Computation for formula unit of Silver nitrateThe given mass of silver nitrate has been 5.50 grams.
The molar mass of silver nitrate has been 169.84 g/mol.
The moles of silver nitrate has been:
[tex]\rm Moles=\dfrac{Mass}{Molar\;mass} \\\\
Moles=\dfrac{5.5}{169.84}\;mol\\\\
Moles=0.032\;mol[/tex]
The moles of silver nitrate in the sample has been 0.032 mol.
The number of molecules of silver nitrate has been:
[tex]\rm 1\;mole=6.023\;\times\;10^2^3\;molecules\\ 0.032\;mol=0.032\;\times\;6.023\;\times\;10^2^3\;molecules\\ 0.032\;mol=1.93\;\times\;10^2^2\;molecules[/tex]
The number of molecules of silver nitrate in 5.5 grams has been [tex]1.93\;\times\;10^2^2[/tex]. Thus, option A is correct.
Learn more about formula unit, here:
https://brainly.com/question/19293051
how many layer does the earth have?
Answers
Answer:
The earth is split into four major layers: the crust, the mantle, the outer core and the inner core
Explanation:
how are living organisms in an ecosystem linked
Answers
Answer:
An environment is a local area of living creatures existing related to the nonliving segments of their current circumstance, interfacing as a framework. These biotic and abiotic segments are connected together through supplement cycles and energy streams.
Explanation:
Answer:
Living organisms require a proper, stable ecosystem/envirornment to function
Explanation:
How is stoichiometry used to calculate energy absorbed when a mass of
solid melts?
O A. Grams solid x mol/g * A Hreaction
B. Grams solid x mol/g * A Hp
C. Grams solid x mol/g * A Hfusion
O D. Grams solid x mol/g * A Hvap
Answers
Answer: Option C is correct
Grams solid x mol/g * A Hfusion
Explanation:
This is because from fusion is associated to melting and this is a process where solid particles or substances goes through transition phase and turn to liquid.
There for the stoichiometry is used to calculate energy absorbed when solid mass melt with the formula Grams solid x mol/g * A Hfusion.
Convert 4.1x10^-4 moles of carbon into atoms.
Answers
Answer:
2.5×10^20atoms
Explanation:
number of moles= number of particles/Avogadro's constant
number of particles=Avogadro's constant × number of moles
number of particles=(6.02×10^23) × (4.1×10^-4)
number of particles=2.5×10^20
How does competition among different species lead to extinction?
Answers
[tex]\huge{\textbf{\textsf{{\color{pink}{An}}{\red{sw}}{\orange{er}} {\color{yellow}{:}}}}}[/tex]
Interspecific competition often leads to extinction. The species that is less well adapted may get fewer of the resources that both species need. As a result, members of that species are less likely to survive, and the species may go extinct.
Thanks Hope it helps.If you have 3 mol of glucose in 6 L of solution, what is the molarity of the
solution?
Answers
Answer:
0.5 M
Explanation:
Molarity = moles of solute / liters of solution
how do some plants grow and survive in the harshest environments like the dessert or under water
Answers
Answer:
Adaptation
Explanation:
Some plants grow and survive in the harshest environments like the desert or underwater by adapting to the environment. For example, some plants in the desert have thick, waxy skin to reduce loss of water and to reflect heat. They also have large, fleshy stems to store water. Also, specific plants like cacti in the desert have spikes to protect them from animals wishing to use stored water. These adaptations help them to grow and survive in the desert.
In the ocean, plants have adapted to absorb all the water and carbon dioxide they need from the water they live in. Just as cacti have adapted to live in brutally hot deserts, ocean plants have adapted to deal with things like ocean tides and the salinity (or salt levels) of the water around them. Many ocean plants cling tightly to rocks in order to avoid being swept away by ocean tides.
Hope this helps! Can I have brainliest?
Mark drove 14 miles EAST and then 23 miles WEST. His displacement from where he started would be
Answers
Answer:
9 miles west
Explanation:
hope this helps
What type of reaction occurs when one element or ion within a compound is exchanged with another element or ion?
combustion
decomposition
single-displacement
double-displacement
Answers
Answer:
When an element replaces another in aone element or ion within a compound is exchanged with another element or ion , the reaction is called
single-displacementWhat is a jet stream. in your own words but be more detailed about what you say.
(don't send a link)
Answers
Scientist introducing a gene to the DNA of these fish that cause the fish to glow an example of which application of biotechnology?
A. Artificial selection
B. Genetic Engineering
C. cloning
D. Artificial intelligence
Answers
Answer:
The correct answer is B
Explanation:
Genetic engineering is an example of which application of because the genes are changed to get the desired features in an organism.
What is genetic engineering?Genetic engineering is the process in which we alter the genetic makeup of an organism to produce desired characterictics in that organism.
So we can conclude that Genetic engineering is an example of which application of because the genes are changed to get the desired features in an organism.
Learn more about here Genetic engineering: https://brainly.com/question/2780091
Zinc reacts with Copper (II) chloride to produce Copper and Zinc chloride. -write chemical formula
Answers
Answer:
here's the answer hope it helps
In 2Fe+3Cl2=2FeCl3, how much iron (Fe) is needed to produce 4 moles of FeCl3?
In 2Fe+3Cl2=2FeCl3, how much FeCl3 is produced when 5 moles of Fe react in excess chlorine?
Answers
Answer:
4 mol Fe
5 mol FeCl₃
Explanation:
Step 1: Write the balanced equation
2 Fe + 3 Cl₂ ⇒ 2 FeCl₃
Step 2: Calculate how much iron (Fe) is needed to produce 4 moles of FeCl₃
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
4 mol FeCl₃ × 2 mol Fe/2 mol FeCl₃ = 4 mol Fe
Step 3: Calculate how much FeCl₃ is produced when 5 moles of Fe react in excess chlorine
According to the balanced equation, the molar ratio of Fe to FeCl₃ is 2:2.
5 mol Fe × 2 mol FeCl₃/2 mol Fe = 5 mol FeCl₃
Consider the following statements about weeding and identify the incorrect one.
a) Weeding is best done during tilling itself.
b) Weeding is the process of growing weed.
c) Weeding is the process of removal of weeds.
d) Weeding is usually done manually or by using weedicides.
Answers
Answer:
b) Weeding is the process of growing weed
Explanation:
Wedding is an agricultural process carried out to ensure maximum you won't do. It is the process by which weeds i.e. unwanted plants are removed.
As rightly stated in the question;
- Weeding is either done by manual means e.g Cutlasses or use of weedicides, which are chemicals.
- Weeding is best done during tiling operations.
The incorrect option is that "Weeding is the process of growing weed" rather it is a process of removing weed.